By Karin Elgar
Abstract
Zinc is an essential trace element and is required for many vital functions, including protein folding, as a co-factor for enzymes, in regulating gene expression, supporting cell membrane structure and cell signalling. It also has antioxidant and anti-inflammatory properties. As such, zinc plays an important role in growth and development, immune function, neurotransmission, vision and reproduction. Zinc deficiency is common, especially in developing countries, and can be due to dietary factors, malabsorption and alcoholic liver disease. Zinc supplementation at appropriate levels is considered safe, and has shown benefits in a wide range of medical conditions, including depression, diabetes, attention deficit hyperactivity disorder, male infertility, and the common cold in children.
Cite as: Elgar, K. (2022) Zinc: a review of clinical use and efficacy. Nutr. Med. J., 1 (3): 46-69.
Affiliation: K. Elgar is with the Nutritional Medicine Institute, London, UK.
Corresponding author: Karin Elgar (email info@karinelgar.com).
Article history: Received 01 August 2021; Peer-reviewed and received in revised form 07 January 2022; Accepted 20 January 2022. Available online 30 September 2022.
Published by: The Nutritional Medicine Institute
Open Access: This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs licence (http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reproduction and distribution of the work, in any medium, provided the original work is not altered or transformed in any way, and that the work is properly cited. For commercial use please contact support@nmi.health
Introduction
Zinc is an essential trace element, and it is needed for growth and development, immune function, neurotransmission, vision and reproduction.1 Zinc can bind to more than 300 enzymes and more than 2000 transcription factors.1 On the cellular and molecular levels, zinc has various functions, including a structural role in protein folding, as a co-factor for many enzymes and in regulating gene expression.2 Zinc is also an important mediator of cellular signalling and supports cell membrane structure.3
Deficiency of zinc was first described in 1961 in the Middle East in men with severe growth retardation whose diets consisted mostly of bread and who also ate large amounts of clay.4 This is thought to be due to diets high in phytates from cereal grains, which hinders the absorption of zinc.4 It has been estimated that up to 17% of the global population may be zinc deficient, mostly in low- and middle-income countries.5 It should be noted that these data were published in 2012, but more recent data are not available.
Whilst signs and symptoms of overt zinc deficiency are well defined, and include growth retardation, hypogonadism (reduced function of the testes in men and ovaries in women), rough skin, poor appetite, mental lethargy and frequent infections, mild zinc deficiency is less specific. However, experiments in humans have shown that an experimental low-zinc diet can lead to a number of biochemical abnormalities, including decreased serum testosterone level, decreased natural killer cell activity, decreased activity of serum thymulin (a zinc-dependent hormone produced by the thymus and important for immune function), hyperammonaemia, decreased taste, decreased visual dark adaptation and decreased lean body mass.4
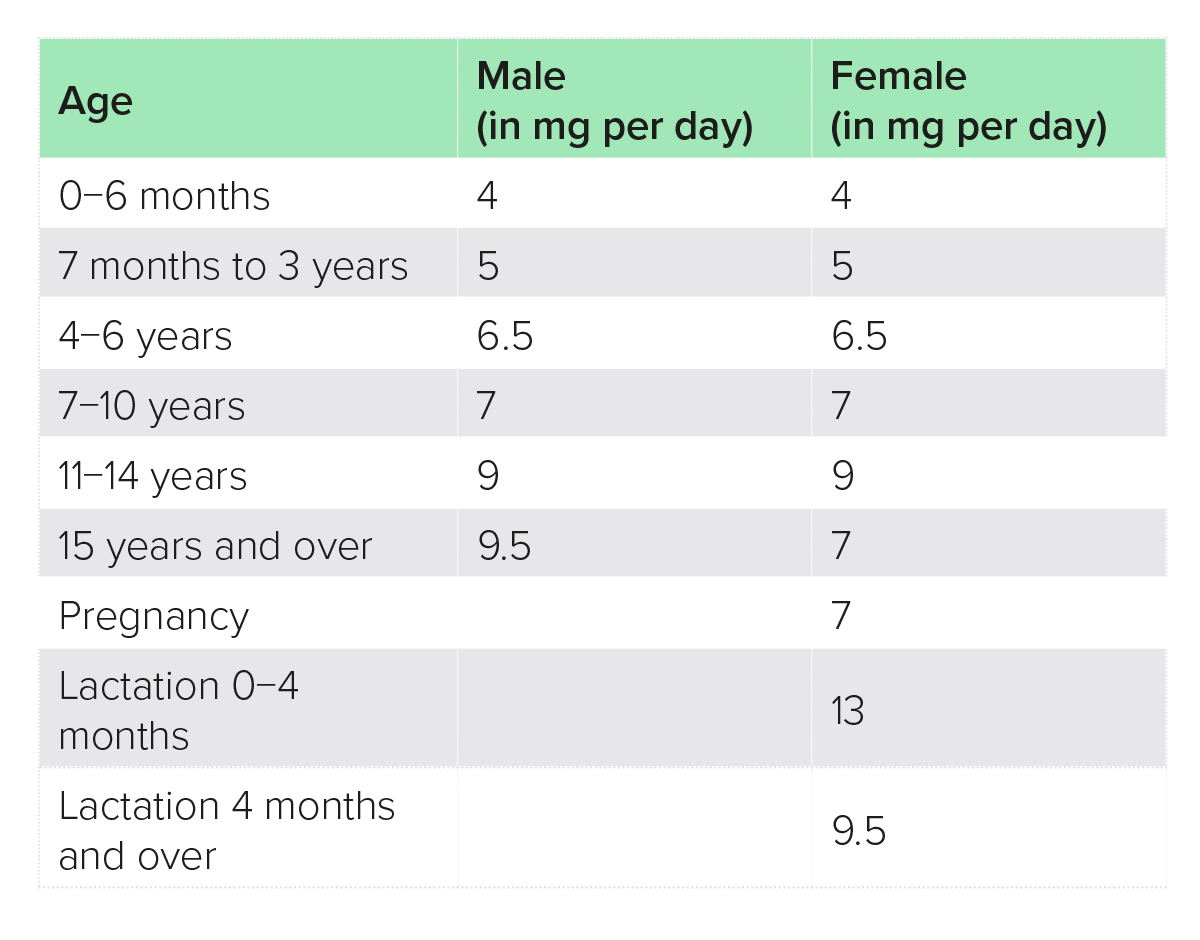
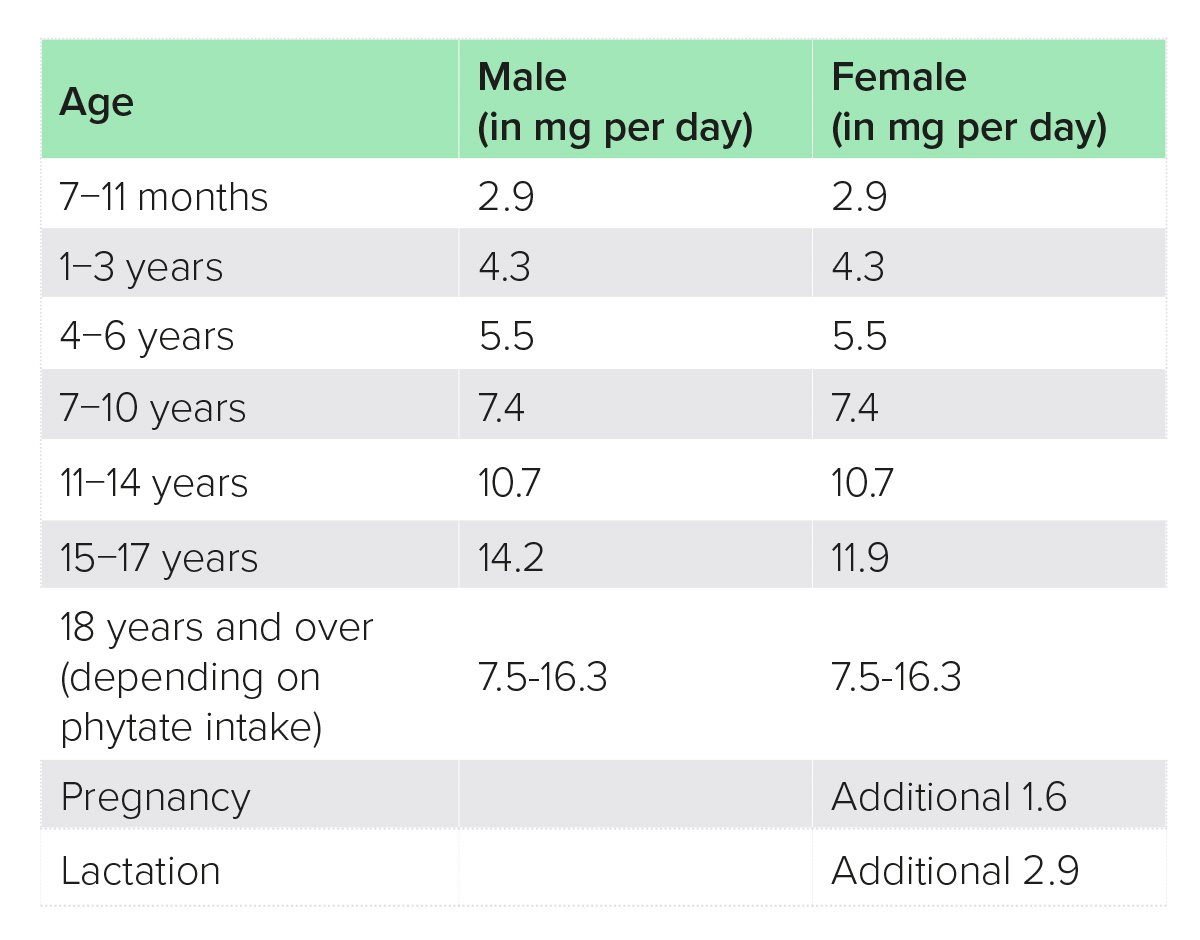
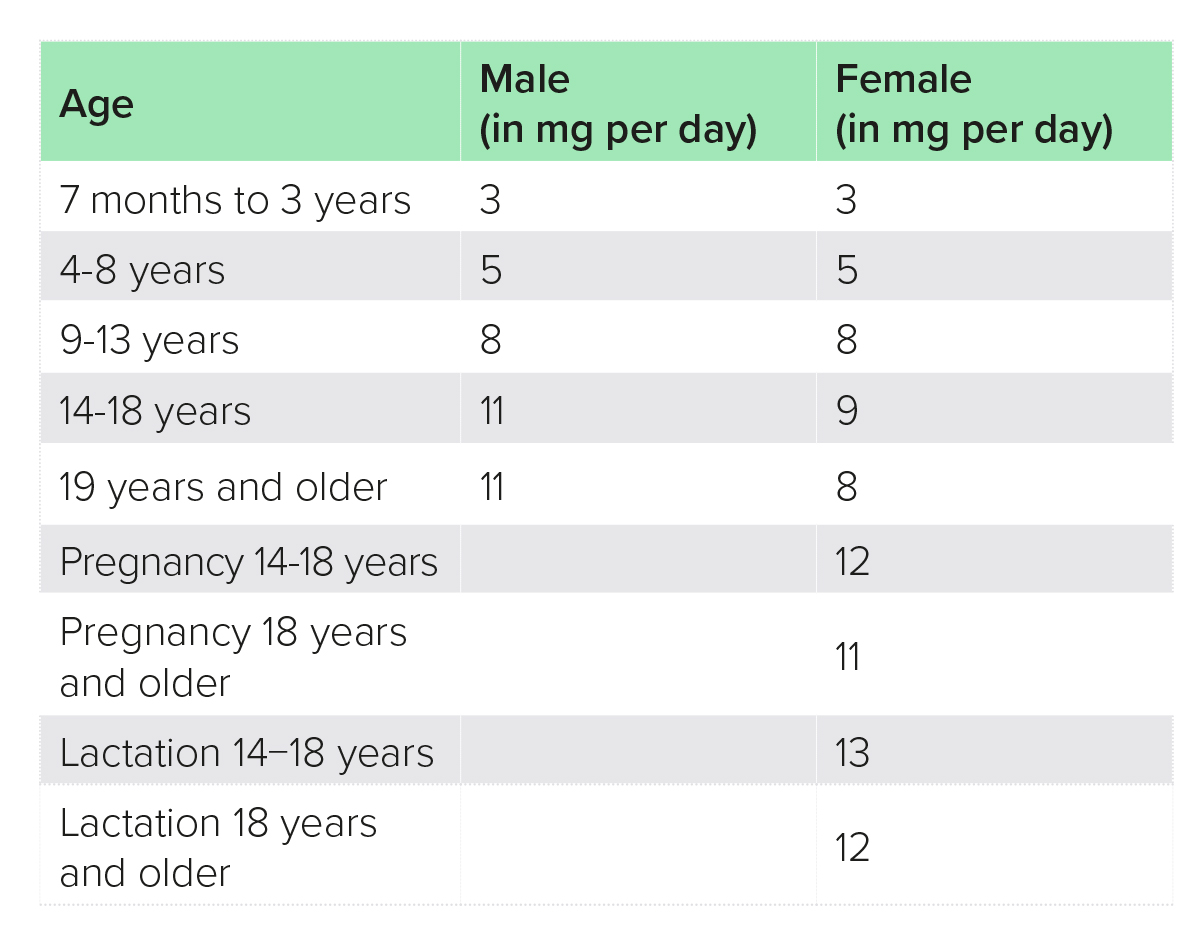
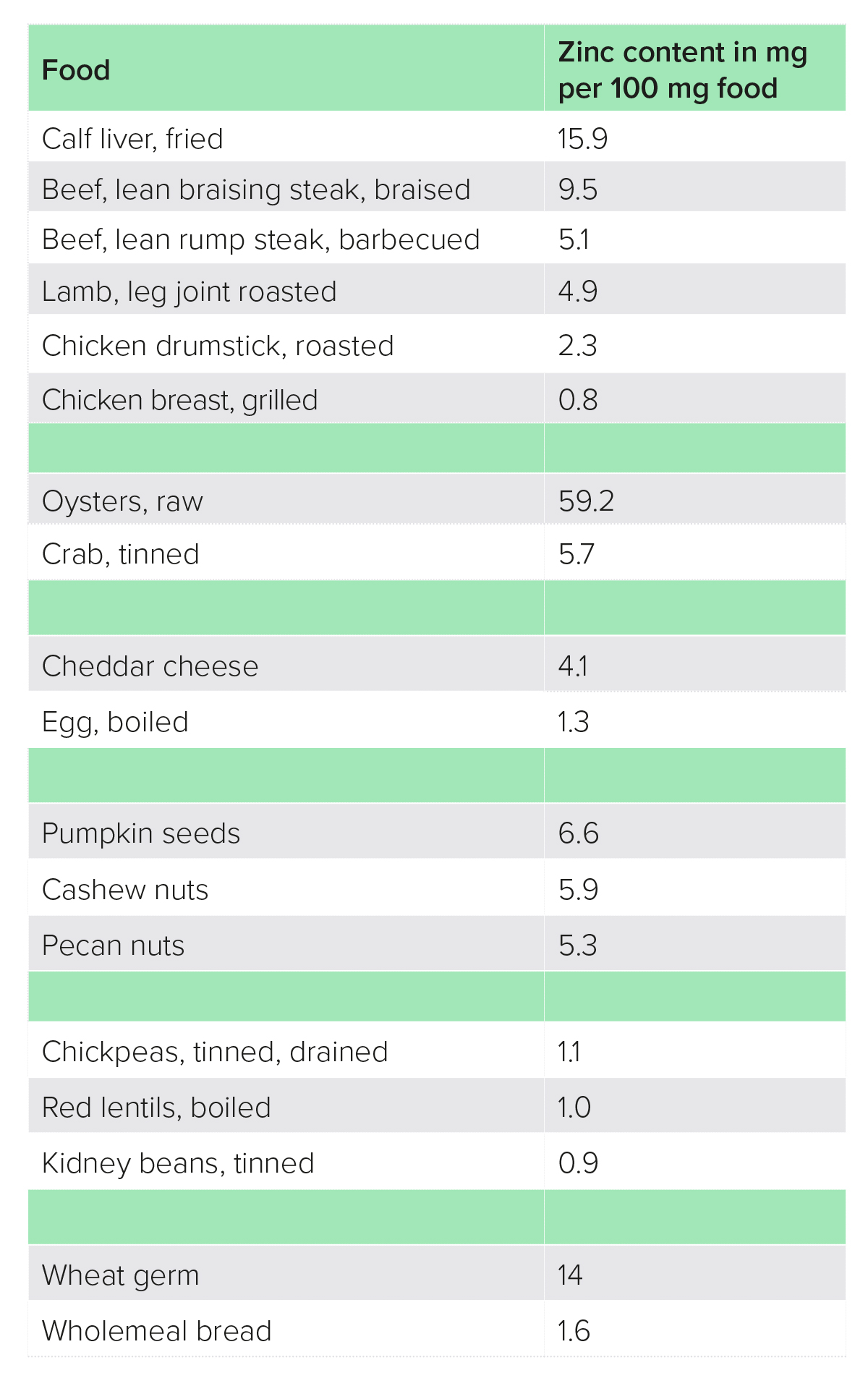
Causes of zinc deficiency include malabsorption [e.g. in inflammatory bowel disease (IBD), unmanaged coeliac disease], alcoholic liver disease and dietary factors (e.g. malnutrition, high intake of phytates).4 The best food sources of zinc are generally speaking protein foods, such as meat, fish, dairy, eggs, pulses, whole grains, nuts and seeds, although absorption from plant sources is lower due to their high phytate content, and it is estimated that vegetarians whose staples are grains and pulses may require up to 50% more dietary zinc (Tables 1−4).2
Table 1: Reference nutrient intakes, UK6
Table 2: Population reference intake, EU7
Table 3: Recommended dietary allowance, USA2
Table 4: Food sources of zinc8
Testing zinc status is complex, and whilst serum or plasma levels are commonly used, these are unlikely to detect mild deficiency as levels are kept within a narrow range through homeostatic mechanisms.2 Other factors can also affect serum or plasma zinc levels, including fasting, inflammation, diurnal rhythm and blood collection issues.9 Depletion studies have shown a dose-dependent response of plasma zinc to zinc supplementation.10
A test that has been suggested for use in nutritional practice is the zinc taste test. This test uses a 0.1% zinc sulphate solution, which should produce a strong metallic taste in a person with adequate zinc levels, but is not tasted in zinc deficiency.11 Whilst this test has been shown to correlate with some other indicators of zinc status, research validating the accuracy of this test is lacking.12,13 Hair zinc levels are also commonly used to evaluate zinc status and have been shown to increase in response to an increase in zinc intake, but the effects of zinc depletion on hair concentrations are not conclusive.7
The aim of this paper is to review the evidence from human clinical trials for the efficacy of zinc supplementation in a range of conditions.
General mechanisms
Antioxidant
Oxidative stress is an important factor in all chronic degenerative conditions, including cancer, cardiovascular disease and neurodegenerative disorders, such as Alzheimer’s disease.3 Zinc has been shown to strengthen antioxidant defences through a number of mechanisms, both directly and indirectly.1,14
Three meta-analyses have summarised the evidence from clinical trials of zinc supplementation on antioxidant biomarkers and found significant benefits of zinc. One meta-analysis of 23 randomised-controlled trials (RCTs) reported significant increases in glutathione (GSH), superoxide dismutase (an antioxidant enzyme) and total antioxidant capacity (TAC), but not glutathione peroxidase.14 A meta-analysis of 21 RCTs found a significant reduction in malondialdehyde (MDA; a marker of oxidative stress),15 and another meta-analysis of 10 RCTs also showed significant reductions in MDA and increases in TAC and GSH, but no effect on nitric oxide (NO).3 All three meta-analyses reported significant inter-trial heterogeneity, and subgroup analyses were inconsistent between biomarkers. The dosage range of zinc across all studies was 11−528 mg per day, and durations 2−48 weeks.
The evidence shows that zinc can lower levels of C-reactive protein (CRP; an inflammatory marker), suggesting an antioxidant effect, although results for other biomarkers of oxidative stress have been inconsistent. The heterogeneity observed may be due to differences in study designs and study populations.
Anti-inflammatory
Like oxidative stress, excessive/inappropriate inflammation is associated with many chronic conditions. Inflammatory processes involve the release of pro-inflammatory cytokines and mediators, such as tumour necrosis factor-alpha (TNF-α) and various interleukins (ILs).16
Several meta-analyses have evaluated the clinical evidence for effects of zinc on inflammatory biomarkers, and consistently showed significant reductions in CRP.15,17,18,19 For other inflammatory markers, the results were inconsistent. Whilst some meta-analyses found reductions in TNF-α,15 others did not find an effect.16,17 Also, some found beneficial effects on IL-6,15,16 others did not.17 All meta-analyses reported heterogeneity amongst study results, but subgroup analyses were inconsistent. Dosages generally ranged between 11 and 50 mg per day, for 2−72 weeks.
These results confirm an anti-inflammatory effect of zinc. The heterogeneity observed may be due to differences in study designs and study populations.
Clinical uses
Acne
Acne is an inflammatory skin disorder affecting more than 85% of teenagers, and is characterised by inflammatory papules, pustules, comedones and sometimes cystic nodules.20 A number of pathological processes contribute to acne, including increased sebum production, aberrant keratinisation of the sebaceous duct of the hair follicle, bacteria such as Cutibacterium acnes, hormonal influences, the skin microbiome and chronic inflammation.20 Both topical and oral zinc have been used in the treatment of acne.
Treatment of acne with zinc was first studied in the 1970s, mostly using 135 mg zinc per day as sulphate for 3 months. Results were mixed, with some finding significant benefits over placebo21 or benefits comparable to tetracycline antibiotics (a standard treatment for acne),22 whilst others did not find significant benefits over placebo23 or zinc to be inferior to antibiotics.24 One uncontrolled, open-label study reported that 13 out of 42 patients stopped zinc sulphate (135 mg per day) due to side-effects, mostly nausea/vomiting and diarrhoea, whilst one patient experienced a perforated pre-existing gastric ulcer during treatment. Only three of the remaining 29 patients had improvements in their acne score after 4 months.25
In 2020, a meta-analysis of 12 observational and 13 intervention studies (which included both oral and topical zinc) found that patients with acne had significantly lower zinc levels than healthy controls.20 Oral zinc significantly improved inflammatory papule count and rate of clinical improvement, but not number of acne pustules. Dosages ranged between 14 and 30 mg as gluconate and 72 and 138 mg as sulphate, for 6−12 weeks.
In 2021, an open-label RCT comparing zinc sulphate, 91 mg per day, versus lymecycline (an antibiotic) for 12 weeks found significant improvements in subjective global acne grading system and acne-specific quality of life (AQOL) in both groups, with improvements in AQOL higher in the zinc group.26
Zinc has also shown benefits in patients with acne in combination with other nutrients: 10 mg zinc per day (as gluconate) with lactoferrin (200 mg per day) and vitamin E (22 IU per day) in one study;27 and zinc 45 mg per day as methionine chelate combined with antioxidants and chromium (390 µg per day) in another study.28
Although there are some contradictory results, overall the evidence suggests a benefit of zinc in patients with acne. Most trials have been carried out with quite high dosages of zinc sulphate (commonly 135 mg per day); lower dosages of a gluconate formulation (10−30 mg per day) have also shown benefits.27,29,30
The mechanisms by which zinc exerts its benefits in acne are thought to be its anti-inflammatory properties, its ability to reduce sebum secretion, inhibiting the activity of androgenetic hormones and antimicrobial activity against C. acnes.26
Attention deficit hyperactivity disorder (ADHD)
ADHD is a common neurodevelopmental disorder characterised by hyperactivity/impulsivity and inattention, which can affect learning, cognition and personal relationships.31
A number of observational studies have yielded conflicting results regarding an association between blood (serum or plasma) and hair zinc levels and ADHD. Two meta-analyses of 22 and 11 studies, respectively, have found no significant difference in zinc status between children and adolescents with ADHD and controls.31,32
Four double-blind, placebo-controlled trials evaluated the efficacy of zinc alongside methylphenidate (Ritalin®), and found that children in the zinc group improved more than those in the placebo group,33,34,35,36 although this was not statistically significant in one study.35 All four studies used zinc as sulphate at dosages between 10 and 22 mg per day, for 6 weeks.
A double-blind, placebo-controlled study using zinc 15 mg or 30 mg per day (as glycinate) alongside amphetamine showed that those in the 30-mg zinc groups needed a 37% lower amphetamine dose to achieve improvements compared with control.37
Only one double-blind, placebo-controlled trial evaluated zinc, 40 mg per day as sulphate, on its own, and showed significant improvements in hyperactive, impulsive and impaired socialisation symptoms, but not in attention deficit symptoms, with better results in children of older age, with high body mass index score, and low zinc and free fatty acid levels.38 Significant improvements were seen from 4 weeks of supplementation.
Two studies reported that adverse events were not significantly different between zinc and placebo groups, except for metallic taste, which was more common in those taking zinc, with 41%36 and 53%,38 respectively, raising the question of adequate blinding.
Overall, the evidence for the use of zinc alongside standard medication in ADHD is consistently positive, with dosages between 10 and 40 mg per day, for at least 4 weeks. Only one study evaluated zinc on its own and also showed positive results with 40 mg zinc per day.
Possible mechanisms include a positive effect of zinc on the metabolism of dopamine,39 as well as its effects on cell membrane stability, antioxidant and hormonal performance.33
Age-related macular degeneration (AMD)
AMD is the leading cause of blindness in the Western world, characterised by loss of central vision resulting in an inability to read, recognise faces or discriminate colours.40 The human retina contains the highest concentration of zinc in the body in women and the second highest in men (after the prostate), and declines with age.41,42
The evidence from clinical trials in reducing the progression of AMD has been mixed, with some showing benefits,41,43,44 and some no benefits.45
By far the largest trial was the Age-Related Eye Disease Study (AREDS), a multi-centre, double-blind, placebo-controlled trial of 3647 participants with AMD with a mean follow-up of 6.3 years.44 Participants were randomised to receive either antioxidants (vitamin C, 500 mg, vitamin E, 400 IU, beta-carotene, 15 mg per day), zinc with copper (80 mg and 2 mg per day, respectively, both as oxide), antioxidants plus zinc with copper, or placebo. The risk of developing advanced AMD was significantly reduced by 28% in the antioxidant plus zinc with copper group, whilst a 25% reduction in risk in the zinc with copper group was not statistically significant. In the high-risk patient group, the combination supplement reduced risk by 34% and zinc/copper alone by 29%, both statistically significant. No serious adverse events were observed.
Overall, the evidence suggests that zinc may reduce the risk of progression of AMD, especially in high-risk patients and combined with other antioxidants. Dosages of 50 mg and 80 mg longer term have shown benefits, and a combination with copper, as in the AREDS study, may be prudent to reduce the risk of a copper imbalance when taking high doses long term.
Reactive oxygen species, oxidative stress and inflammation play an important role in the development of AMD, and the antioxidant and anti-inflammatory properties of zinc are thought to explain its benefits.41
Asthma
Asthma is a chronic inflammatory disorder of the respiratory tract, characterised by airway constriction, inflammation and bronchial hyperresponsiveness, and symptoms including recurrent coughing, wheezing, dyspnoea (shortness of breath) and chest tightness.46,47
Two meta-analyses evaluated zinc levels and risk of asthma with contradictory results. Whilst a meta-analysis that looked at only children found no association,47 another one that included children and adults found that people with asthma had lower blood levels of zinc.46
Two double-blind, placebo-controlled trials have shown benefits of zinc supplementation in children. In one study, severity of asthma in children admitted to hospital for an exacerbation decreased more rapidly with zinc, 15 mg (as bis-glycinate) twice a day, after 24 and 48 hours compared with placebo.48 Fifty-seven percent of children in this study were zinc deficient at baseline. In the other study, children with low zinc status and who were on inhaled steroids were given zinc, 50 mg per day (formulation not reported), for 8 weeks, which led to significant improvements in clinical symptoms compared with placebo.49 Zinc status was defined by serum zinc concentrations in both studies.
Zinc supplementation appears to be of short- and long-term benefit in children, at least those with low zinc levels, although data are too limited to recommend a particular dose. There are no clinical trials in adults.
The antioxidant, anti-inflammatory and immune-modulating properties of zinc are thought to mediate its benefits in asthma.50
Atopic dermatitis
Atopic dermatitis is a chronic inflammatory condition of the skin, characterised by itching and redness. As zinc is important for skin health and skin disorders are observed in zinc deficiency, there has been interest in zinc supplementation in atopic dermatitis.51
A meta-analysis of observational studies showed that atopic dermatitis is associated with lower zinc levels [serum, red blood cells (RBCs) and hair], but two intervention trials gave contradictory results. Whilst one found significant benefits of zinc, 12 mg per day (formulation not reported), for 8 weeks alongside antihistamines and topical moisturiser,51 another study found no benefit with 43 mg per day (as sulphate) for 8 weeks.52 It should be noted that the former study was not placebo controlled, whilst the latter was a double-blind, placebo-controlled study.
At this point, there are insufficient data to confirm the benefits of zinc in atopic dermatitis.
Bone health
Zinc plays an important role in the growth and maintenance of healthy bones, and epidemiological studies have shown that patients with osteoporosis have lower serum zinc levels than healthy controls, and that dietary zinc intake is inversely correlated with fractures.53
Clinical trials have been carried out in a variety of settings, and have shown benefits of zinc supplementation for bone health, markers of bone turnover and/or bone mineral density in patients with osteoporosis and zinc deficiency,54 patients with thalassaemia (an inherited condition leading to anaemia) and low bone mass,55 healthy male volunteers,56 and postmenopausal women with rheumatoid arthritis and osteoporosis,57 but not in healthy pubertal girls58 or patients on haemodialysis.59 Dosages have ranged from 15 to 68 mg per day, with dosages of 25 mg per day or more showing benefits, and formulations have varied widely in these trials.
Overall, the evidence suggests a benefit of zinc for bone health, at least in some populations, at dosages of at least 25 mg per day for at least 3 months.
Cardiometabolic disorders
Cardiometabolic disorders, including heart disease, type 2 diabetes mellitus (T2DM) and metabolic syndrome, are the leading cause of deaths worldwide.60 Zinc is known to be involved in insulin/glucose homeostasis, lipid metabolism and regulating inflammation, important underlying causes of cardiometabolic disorders.60
Cardiovascular risk factors
Cardiovascular risk factors include abnormal blood lipids, dysregulated glucose/insulin metabolism and hypertension. The effect of zinc supplementation on these risk factors has been assessed by a number of meta-analyses, which showed that zinc improves blood lipids and glycaemic control.60,61,62 One meta-analysis compared low-dose (< 25 mg per day) versus high-dose (≥ 25 mg per day) and short-term (< 12 weeks) versus long-term (≥ 12 weeks) supplementation, and found low-dose, long-term supplementation to be of most benefit.60
A meta-analysis of epidemiological studies found patients with hypertension to have significantly lower zinc status, as determined by serum zinc level, than healthy controls.63 The results from intervention trials, however, are conflicting, with one meta-analysis of four RCTs finding no statistically significant improvement in blood pressure,61 whilst another meta-analysis of nine RCTs showed significant improvements with zinc supplementation in systolic, but not diastolic, blood pressure.64 The reasons for this discrepancy are unclear, but are unlikely to be due to heterogeneity of the included RCTs, as the latter meta-analysis found significant heterogeneity for diastolic but not systolic blood pressure whilst the former found no heterogeneity for either. Baseline zinc level may play a role, three of the four studies of the former meta-analysis reported normal baseline zinc levels, whilst the latter did not report on zinc status.
Whilst the evidence for zinc supplementation in hypertension is conflicting, there appears to be a clear benefit with regards to blood lipids and glycaemic control, especially with low-dose, long-term supplementation.
Diabetes, prediabetes and metabolic syndrome
A meta-analysis of epidemiological studies showed T2DM to be associated with lower blood zinc levels compared with healthy controls, which cannot be explained by lower zinc intakes.65 In another meta-analysis, the same researchers also found that whilst moderately high dietary zinc intake reduced the risk of developing T2DM, elevated plasma/serum levels were associated with an increased risk, although there was significant heterogeneity between the studies, with some showing an increased and others a decreased risk with higher plasma/serum levels.66
Four meta-analyses have been conducted over the past 2 years, and all reported benefits of zinc supplementation for glycaemic control and blood lipids in patients with diabetes or prediabetes.17,67,68,69 Dosages have varied widely between 4 and 660 mg per day, and durations from 3 weeks to 1 year.
Improvements in blood lipids, glycaemic control and obesity indices have also been seen in Iranian children with obesity and metabolic syndrome who received zinc, 20 mg per day (formulation not reported), for 8 weeks.70,71
The evidence from clinical trials is in favour of zinc supplementation in T2DM, prediabetes and metabolic syndrome, with benefits for glycaemic control as well as blood lipids. A wide range of dosages have shown beneficial results, making it difficult to suggest a particular dose.
Cognition
Zinc is important for neuronal signalling and is found in high levels in the brain, in particular in areas involved in learning and memory.72
Studies that evaluated cognition in children are covered under children/mental development.
Two double-blind, placebo-controlled studies showed no benefit of zinc on cognition in elderly people. The abovementioned AREDS study [see section ‘Age-related macular degeneration (AMD)’] found no effect of zinc plus copper supplementation (80 mg and 2 mg per day, respectively, both as oxide) on any of six cognitive tests.73 The other trial, in healthy people over 55 years old, compared zinc, 15 mg and 30 mg per day (as gluconate), for 6 months with placebo, and found that out of eight parameters one improved and one deteriorated with zinc, but effects were significant at 3 months only.74
At present the evidence suggests that zinc is not effective in improving cognitive function.
Depression
Depression is a leading cause of disability globally and contributes significantly to the global burden of disease.75 Two meta-analyses showed that people with the highest intake of zinc had a reduced risk of depression, by 28%76 and 33%,77 respectively.
Over the past 2 years, three meta-analyses, of three, five and eight trials, respectively, assessed zinc supplementation trials and found significant improvements in depressive symptoms.75,76,78 Dosages ranged from 7 to 220 mg per day, with the most commonly used dose being 25 mg per day, and durations ranged from 2 to 12 weeks, with most trials lasting 12 weeks. None of the meta-analyses reported effects of dose or duration on outcomes.
The evidence suggests that zinc is beneficial in reducing depressive symptoms in depressed patients, with a dose of 25 mg per day for 12 weeks being the most commonly used regimen.
Several possible mechanisms to explain the beneficial effects of zinc in depression have been discussed, including its effects on the N-methyl-D-aspartate (NMDA) and gamma-aminobutyric acid (GABA) receptors, which are thought to be involved in the development of depression, regulation of serotonin metabolism via its anti-inflammatory effects and its involvement in regulating brain-derived neurotrophic factor, which is important for neuroplasticity and memory.75,77,78
Fertility (male)
Seminal fluid is high in zinc and is thought to play an important role in sperm function.79 Two meta-analyses of observational studies have shown seminal zinc levels to be significantly lower in infertile compared with fertile men.79,80
A meta-analysis of five intervention trials found significant improvements with zinc supplementation in semen volume, sperm motility and the percentage of normal sperm morphology, but not sperm viability, sperm concentration, sperm count or percentage of abnormal sperm morphology.79 Zinc dosages ranged from 15 to 100 mg per day for 45 days to 20 weeks, with 100 mg per day (as sulphate) appearing most beneficial.
The evidence suggests that zinc supplementation is beneficial for male fertility at a dose of 100 mg for at least 45 days.
It is thought that the antioxidant properties of zinc play an important role in its beneficial effect on male fertility.81
Gastric ulcerhttps://www.who.int/elena/titles/bbc/zinc_diarrhoea/en/
Gastric and duodenal ulcers are a disruption of the mucous layers that lead to inflammation.82 In view of its importance in healing, zinc has been of interest and has shown promise in animal experiments.83
Zinc acexamate is has been marketed in Taiwan, Spain and a number of South American countries. In Spain it is licenced for the treatment and prevention of gastric and duodenal ulcers.84 In the 1980s, 18 clinical trials were published on the effect of zinc acexamate, 12 of which were subject to a meta-analysis in 1992 that showed the zinc supplement was significantly more effective than placebo and as effective as an H2-blocker in improving gastric ulcers.85 All but one study used 900 mg per day (elemental weight not reported), the other study used 300 mg per day, and study duration ranged from 3 to 6 weeks.
In the 1970s, a double-blind, placebo-controlled trial of 18 patients with gastric ulcer confirmed by barium meal (before and after treatment) using zinc as sulphate, 150 mg per day for 3 weeks, found a three times higher healing rate in the zinc-treated compared with the placebo-treated patients.86 However, two more double-blind, placebo-controlled trials found no benefits with lower dosages of zinc sulphate, 50 mg per day83 or 50 mg every other day,82 alongside standard treatment, compared with placebo.
The evidence is in favour of zinc acexamate as an effective treatment for gastric ulcers, whilst it is unclear whether the conflicting results with zinc sulphate are due to differences in dose or other variables.
Inflammatory bowel disease (IBD)
IBD are chronic inflammatory conditions of the gastrointestinal (GI) tract, and include ulcerative colitis (UC), which affects the colon only, and Crohn’s disease (CD), which can affect any part of the GI tract.87
Two large cohort studies of 170 776 women found that zinc intake was inversely related to the risk of developing CD, but not UC, and the association was stronger for dietary than supplemental intake.88
A double-blind, placebo-controlled trial of zinc, 150 mg per day (as sulphate) for 4 weeks, alongside standard treatment, in 51 patients with UC had no benefits over placebo.89 Another study, supplementing zinc, 35 mg per day (as gluconate) for 2 months in patients with low zinc levels, found significant improvements in IL-10 and IL-2, but not other ILs, as well as improvements in the Mayo disease score, versus control group, which were patients with UC with normal zinc levels who received placebo.87
Three clinical trials looked at various outcome measures in patients with CD in remission, and found improvements in intestinal hyperpermeability,90 RBC status of polyunsaturated fatty acids (linoleic acid, arachidonic acid and omega-3 fatty acids)91 and thymulin activity.92 All three studies used zinc sulphate at dosages of 46 mg per day for 6 weeks91 or 3 months,92 or 75 mg per day for 8 weeks.90
The evidence in UC is contradictory, whilst in CD improvements in a number of disease parameters have been observed. As all patients with CD were in remission, it is difficult to make conclusions regarding the therapeutic benefits of zinc.
Liver disease
It is estimated that, in the UK, liver disease is the third most common cause of premature death, and mortality has increased by 400% since 1970.93 Zinc deficiency is very common in patients with liver disease, with up to 83% of patients with liver cirrhosis being zinc deficient.93
A review and meta-analysis of 12 RCTs found no effects of zinc supplementation (dosages not reported) on chronic hepatitis C, cirrhosis or serum albumin levels, but a significant benefit for hepatic encephalopathy (HE), based on three studies.94 Another meta-analysis that reviewed seven RCTs also reported benefits of zinc alongside therapy with lactulose in HE, with a dosage range of 25−180 mg per day, which was administered for 6 months in most studies.95
A meta-analysis of four intervention trials found that zinc supplementation, dosage range 3.4−214 mg per day, had no effect on mortality from cirrhosis at 6 months.93
A recent double-blind, placebo-controlled trial in 56 obese/overweight patients with non-alcoholic fatty liver disease (NAFLD) found significant improvements in glycaemic control, oxidative stress and liver enzymes, but not liver steatosis or fatty liver index with zinc gluconate, 30 mg per day for 3 months, alongside a calorie-reduced diet.96,97
Overall, the evidence suggests that zinc supplementation is of benefit in HE and NAFLD (based on only one study), but not cirrhosis or hepatitis C.
The mechanism by which zinc improves HE is thought to be its role in ammonia metabolism, which is disrupted in patients with HE.95 The benefits of zinc for NAFLD are thought to be mediated through its effects as an antioxidant, and by improving glycaemic control and lipid metabolism.96
Mucosal health
Intestinal permeability
Zinc deficiency has been shown to cause damage to the gut membrane through inflammatory cell infiltration, and patients with chronic disturbances of intestinal permeability have reduced levels of mucosal zinc.98 In a small randomised crossover study in 10 healthy volunteers, 70 mg zinc per day as carnosine, for 5 days, prevented indomethacin-induced increases in intestinal permeability.99
Zinc has also been shown to have beneficial effects on intestinal permeability in patients with CD [see section ‘Inflammatory bowel disease (IBD)’ for details].
Whilst clinical evidence is scarce, zinc appears to be beneficial in supporting the integrity of the intestinal mucosa.
Oral mucositis (OM)
OM refers to ulcerative lesions in the oral mucosa, and is a common side-effect of chemo- and/or radiotherapy.100
A meta-analysis of 10 RCTs (nine with oral administration and one using a zinc mouthwash) found significant benefits in severity of OM, as well as delayed onset and faster healing, but no reduction in incidence, in patients receiving chemo- and/or radiotherapy.100 Zinc dosages ranged from 21 to 150 mg per day.
Since then, three more studies have shown benefits with zinc supplementation in both adults101 and children.102,103
The evidence suggests that zinc is of benefit in patients receiving chemo- and/or radiotherapy in reducing severity of OM, with zinc dosages of 21 mg per day (as sulphate) in adults and 7 mg per day (as gluconate) in children showing beneficial results.
The antioxidant and anti-inflammatory properties of zinc may play a role in its effects on OM.104
Recurrent aphthous stomatitis (RAS)
RAS is characterised by recurrent ulcers of the oral mucosa that generally heal by themselves within 10 days, and affects about 25% of the general population. The underlying causes are unknown.105 A meta-analysis of 19 case−control studies found that patients with RAS had significantly lower levels of zinc than healthy controls.105
Two studies using oral zinc sulphate have shown benefits in both treatment and prevention of RAS, with dosages of 69 mg per day for 12 weeks106 and 50 mg per day for 1 month (both as sulphate).107 A mucoadhesive formula of zinc sulphate, providing 5 mg three times per day, has also shown to improve lesion size from day 3 and pain from day 5 compared with placebo.108 One study from 1982, however, showed no benefits (article not accessible, no details provided in abstract).
A study from 1977 in 32 patients with RAS found that all of those who had low zinc levels at baseline improved with zinc supplementation (up to 150 mg per day as sulphate, duration not reported), whilst only three of eight patients with normal zinc levels improved.109
Overall, the evidence suggests that zinc is of benefit in RAS, but this may depend on zinc status. A regimen of 50 mg per day (as sulphate) for at least 1 month has shown benefits.
Polycystic ovary syndrome (PCOS)
PCOS is a common endocrinological disorder with gynaecological, metabolic and psychological abnormalities, including high testosterone levels, hirsutism (male pattern hair growth), anovulation, infertility, menstrual disturbance, insulin resistance, obesity and mood swings.110
Two double-blind, placebo-controlled trials in women with PCOS found significant improvements in biochemical markers and symptoms with zinc, 50 mg per day (as sulphate) for 8 weeks: one found improvements in glycaemic control and lipid profiles,111 whilst the other found improvements in alopecia, hirsutism and MDA, but not on hormone profiles, other oxidative stress markers or inflammatory cytokines.112
A study that used zinc, 8 mg per day, alongside magnesium (200 mg per day), calcium (800 mg per day) and vitamin D (400 IU per day) also found significant improvements in glycaemic control and blood lipids.113
Whilst the number of clinical trials is limited, they suggest a benefit of zinc supplementation for women with PCOS; 50 mg of zinc per day (as sulphate) has shown benefits within 8 weeks.
The positive effects of zinc on insulin homeostasis, lipid metabolism, improving antioxidant status and regulating inflammation60 are likely to explain its benefits in PCOS.
Pregnancy
Due to growth taking place, the need for zinc is increased during pregnancy.2 A 2021 Cochrane review and meta-analysis found no significant benefits for preterm birth, stillbirths, perinatal deaths or birth weight with zinc supplementation compared with controls.114
Gestational diabetes mellitus (GDM)
Two double-blind, placebo-controlled trials evaluated the benefits of 30 mg per day zinc (as gluconate) for 6 weeks in women with GDM, and found significant benefits in glycaemic control, high-sensitivity (hs)-CRP, TAC and some but not all blood lipids, but not in NO, GSH, MDA or pregnancy outcomes.115,116 Similar benefits have been seen with zinc, 8 mg per day (formulation not reported), alongside magnesium (200 mg per day), calcium (800 mg per day) and vitamin D (400 IU per day) for 6 weeks.117
An RCT in women with gestational impaired glucose tolerance, however, found no statistically significant benefits for glycaemic control with zinc, 30 mg per day (as gluconate) for 8 weeks.
Although research is limited, it appears that zinc, either alone or with other nutrients, has beneficial effects on glycaemic control in women with GDM. A dose of 30 mg zinc per day (as gluconate) for 6 weeks has shown benefits.
Respiratory infections
Common cold
The common cold is usually a mild illness that does not progress to serious outcomes, such as pneumonia or requiring hospitalisation, but presents a significant socioeconomic burden.118 Zinc plays an important role in immunity, and has therefore been studied in the prevention and management of the common cold.
Harri Hemilä has conducted three meta-analyses of zinc lozenges in the common cold in adults, all three finding a shorter duration of colds compared with placebo treatment.119,120,121 The most recent meta-analysis that included five RCTs found a 33% shorter duration with zinc lozenges with dosages of 80−207 mg per day, although dosages of > 100 mg per day had no additional benefit.121
However, the validity of the matching between active lozenge and placebo was questioned as early as 1987, suggesting that the taste of the zinc lozenge may have unmasked the blinding.122 In 2020, Harri Hemilä et al. carried out a double-blind, placebo-controlled trial of zinc lozenges (as acetate), 78 mg per day for 5 days, starting at the onset of symptoms of the common cold, versus placebo, which was matched for taste and appearance.123 There was no significant difference in duration of cold symptoms between the zinc and the placebo lozenges. It is unclear whether this was due to the lower dose as compared with those evaluated in the above meta-analyses.
At present, the evidence is unclear as to whether zinc lozenges are beneficial in adults with the common cold or whether benefits reported in some studies are due to a placebo effect.
Zinc supplementation has also been evaluated in children with the common cold. Four studies showed a shortening of duration,124,125,126,127 whilst two studies did not.128,129 One of the studies that did not find a shorter duration, however, reported that symptoms were less severe from day 2 of treatment compared with placebo.128 A reduced severity has also been reported in another study.125 Of four studies that looked at preventive use of zinc, three reported a significantly lower frequency of colds in those treated with zinc,125,126,127 whilst the fourth did not see a reduction in incidence.124
Not all studies reported dose of zinc, where it was reported it ranged from 15 mg per day for prophylaxis to 60 mg per day for treatment. A variety of formulations was used and did not affect results.
Although there are contradictory results, in children, zinc appears to be efficacious in preventing colds as well as reducing the duration, at a dose of 15 mg per day for prevention and 30−60 mg per day for treatment, started within 24−48 hours of first onset of symptoms.
Apart from its general effects on immunity, zinc has been shown to inhibit rhino-viruses,130 which cause about 50% of common colds.131
Pneumonia
Pneumonia causes 15% of deaths in children under 5 years old worldwide,132 mostly in developing countries where zinc deficiency
is common.
Three meta-analyses evaluated zinc as an adjunct to treatment of pneumonia in children under 5 years old, in low- and middle-income countries, covering similar sets of studies.133,134,135 No effect on treatment failure, change of antibiotic therapy or time to recovery was seen in any of them. A significant 57% reduction in mortality was reported in one meta-analysis,134 whilst mortality reductions in the other two meta-analyses (36%133 and 31%135) did not reach statistical significance.
A Cochrane review and meta-analysis of six RCTs concluded that zinc supplementation significantly reduced the incidence and prevalence of pneumonia in children under 5 years old.136 All studies included in this review were from low- and middle-income countries.
Zinc supplementation appears to be beneficial for the prevention of pneumonia in young children and may reduce mortality. However, this is in the context of geographical areas where zinc deficiency is common, and can therefore not necessarily be extrapolated to children in Europe or the USA.
Sleep
A number of epidemiological studies have shown an association between zinc status and sleep, and animal studies have shown benefits of supplemental zinc for sleep.137
Three double-blind, placebo-controlled trials have investigated the effects of zinc supplementation on sleep. In a study of 120 healthy adults, both a zinc-rich diet and a yeast-based zinc supplement (both containing 15 mg zinc per day) for 12 weeks showed significant improvements of sleep-onset latency and sleep efficiency with zinc compared with placebo.138 A study in 54 intensive care unit nurses also found significant improvements in sleep quality with zinc, 50 mg every 3 days (as sulphate) for 1 month.139 A study in young women with premenstrual syndrome, on the other hand, showed no benefits for sleep parameters with 30 mg zinc per day (as gluconate) for 3 months, although a significant benefit on physical aspects of quality of life was seen.140
At present, the evidence for the use of zinc to improve sleep is limited and mixed. A low dose of zinc (15 mg per day) may be of benefit.
Possible mechanisms linking zinc and sleep include its effects on neurotransmitters and their receptors in the brain.137
Taste
As mentioned above, zinc is important for our sense of taste and smell, and zinc deficiency is associated with dysgeusia (altered taste perception).141 Dysgeusia can also occur as a side-effect, in particular from chemo- and/or radiotherapy, affecting approximately 56% of patients on chemotherapy alone and 76% of those on combined chemo- and radiotherapy, with large variations with cancer site and type of treatment.142
A meta-analysis of four RCTs in patients undergoing radiotherapy for head and neck cancers found that zinc supplementation decreased the incidence of dysgeusia by 28%, but did not improve taste acuity more after radiotherapy than controls.143 Dosages ranged from 72 to 150 mg per day.
Studies in patients receiving chemotherapy have been mixed, with two showing no significant benefits,144,145 and one showing a reduction in the duration of dysgeusia.146 The study that found significant benefits used a specific formulation, β-alanyl-L-histidinato zinc, 33 mg per day, whilst the others used zinc sulphate.
A number of studies also investigated the effect of zinc supplementation in dysgeusia due to other reasons. Two studies, one in adults147 and one in children,148 found no benefits of zinc in patients on haemodialysis. Similarly, studies in elderly people found no improvements in taste with zinc supplementation.149,150
Most studies in zinc-deficiency-related or idiopathic (of unknown cause) dysgeusia have found beneficial effects with zinc supplementation, especially with the β-alanyl-L-histidinato zinc formulation (34 and 68 mg per day),151,152,153 with zinc gluconate,154,155 an Ayurvedic zinc formulation156 and with zinc picolinate.157 However, another study found no benefits, using zinc sulphate.158
Zinc supplementation appears to be of benefit in radiotherapy-induced, idiopathic and zinc-deficiency-induced taste disorders, but evidence is mixed for chemotherapy-induced dysgeusia, and negative for taste abnormalities associated with haemodialysis and in elderly people. Whether or not zinc supplementation is beneficial also appears to depend on the formulation, rather than the dose, with all studies using β-alanyl-L-histidinato zinc and most using zinc gluconate showing benefits.
Although the exact mechanisms are unclear, it has been suggested that a zinc-dependent salivary enzyme, gustin, is crucially involved in taste sensation, and zinc is also important in the regeneration of taste bud cells.159
Thyroid
Zinc is involved in thyroid metabolism in various ways, including as a co-factor of the deiodinase enzymes that convert the less active thyroxine (T4) to the more active triiodothyronine (T3),160 the binding of T3 to nuclear receptors,161 and in the formation of thyrotropin-releasing hormone.162
In 2021, a systematic review evaluated both epidemiological and intervention studies, and concluded that the evidence from studies is contradictory for both an association between zinc levels and thyroid function and benefits of zinc supplementation.163 Four of the eight intervention studies reviewed were in zinc-deficient people with Down’s syndrome, with two of them showing an increase in thyroid-stimulating hormone (TSH) and two showing a decrease.163
Two RCTs looked at hypothyroid patients and found beneficial effects of zinc either alone or in combination with other nutrients. In one study of 68 overweight or obese hypothyroid women, zinc (30 mg per day as gluconate) was given alone and in combination with selenium (200 µg per day) for 12 weeks and compared with placebo.160 Both the combination of zinc and selenium and zinc alone increased free T3 levels compared with placebo and selenium alone, with zinc alone being significantly more effective in raising free T3 than zinc plus selenium. Total T3, free and total T4 and TSH were not significantly different between groups after 12 weeks. In the other study, zinc was given with magnesium (250 mg per day) and vitamin A (25 000 IU twice per week) for 10 weeks, and significant improvements in free T4, weight and hs-CRP were observed compared with placebo.164 Patients in both studies were on levothyroxine.
At present, the evidence does not suggest a benefit of zinc supplementation for thyroid function in people with Down’s syndrome. In hypothyroid patients, limited research suggests a benefit both on its own and in combination with other nutrients at a dose of 30 mg per day (both studies used a gluconate formulation).
Wound healing
Zinc plays an important role in wound healing through its effects on immunity, cell proliferation, and through protein and DNA synthesis.165 Zinc is also involved in the regulation of growth factors that are involved in anabolic response and healing.166 Both topical and oral zinc have been used in a number of conditions related to wound healing, including pressure and leg ulcers.
Pressure ulcers
Pressure ulcers are areas of damage to the skin and underlying tissue, and are common in elderly people, affecting up to 32% in acute and long-term settings.141,168
A review and meta-analysis of seven clinical trials, three on oral and four on topical zinc, found a significant benefit of zinc in the healing of pressure ulcers.169 All three studies on oral zinc showed benefits (doses not reported).
Two more studies, using zinc (zinc 30 mg per day, formulation not specified) in combination with L-carnosine (116 mg plus 34 mg zinc),170 and in combination with arginine (9 g) and vitamin C (500 mg),171 reported benefits of zinc for the healing of pressure ulcers. However, a retrospective study from 2001, using 100 mg zinc per day (as sulphate), found only minor benefits but significant side-effects, with patients on zinc having 7.8 times the risk of getting an infection requiring antibiotics and 12.5 times more likely to experience nausea/vomiting.167 Side-effects lessened when a low-dose multivitamin and mineral supplement was given concurrently, possibly by alleviating the effects high-dose zinc may have had on copper levels.
Overall, zinc appears to be beneficial for pressure ulcers, with dosages of 30−34 mg per day showing benefits.
Apart from the above-mentioned mechanisms, positive effects on Langerhans cells (macrophages in the skin) have also been observed in patients with pressure ulcers.172
Leg ulcers
A number of trials in the 1970s investigated the potential benefits of oral zinc in the treatment of leg ulcers, but unfortunately not all publications have been accessible. Whilst one study from 1970 showed that patients with leg ulcers had lower zinc levels than healthy controls and improved healing with zinc supplementation,173 all other accessible early-intervention trials found no benefit of oral zinc.174,175,176,177
A more recent double-blind, placebo-controlled study in patients with diabetic foot ulcers found significant benefits with zinc, 50 mg per day as sulphate for 12 weeks.178
Whilst limited evidence suggests that zinc supplementation is of benefit for diabetic foot ulcers, the accessible evidence does not support a benefit for leg ulcers.
Children
Zinc deficiency is amongst the most common nutrient deficiencies globally, and affects children in many low- and middle-income countries where dietary patterns rely heavily on high-phytate foods.179 Zinc is essential for cellular growth due to its direct impact on nucleic acid and protein synthesis, hormonal mediators of growth, risk of infection and appetite, and is thought to be an important factor in childhood stunting.180 Zinc deficiency may contribute to childhood mortality and ill health.
A number of meta-analyses evaluated the effect of zinc supplementation on growth in children under 5 years old with slightly conflicting results. Whilst some found no effects,180 others found significantly better growth indices with zinc compared with controls,179,181 although the effect sizes were generally small. These conflicting results may be due to different study populations and baseline zinc status, as well as other factors that are known to contribute to stunting such as general malnutrition, poor maternal health/nutrition, frequent infections, especially diarrhoea, and poor feeding and care early in life.182
Three meta-analyses also looked at motor and mental development, and found no significant effect of zinc supplementation.72,183,184
In developing countries, diarrhoea is a common cause of death in young children. The World Health Organisation recommends routine use of zinc supplementation, at a dosage of 20 mg per day for children older than 6 months or 10 mg per day in those younger than 6 months, for 10–14 days, alongside oral rehydration salts.185 A Cochrane review in 2016 concluded that “In areas where the prevalence of zinc deficiency or the prevalence of malnutrition is high, zinc may be of benefit in children aged 6 months or more. The current evidence does not support the use of zinc supplementation in children less than 6 months of age, in well-nourished children, and in settings where children are at low risk of zinc deficiency.”186
A 2021 meta-analysis of 23 RCTs in low- and middle-income countries found that zinc supplementation in children under 5 years old significantly decreased all-cause mortality by 16%, and death from pneumonia, infection and diarrhoea by 30%, 46% and 15%, respectively.187
The evidence presented here for supplementation in children refers mostly to low- and middle-income countries where zinc deficiency is common, and cannot necessarily be used to inform supplementation of young children in developed countries.
Safety in children
Zinc is safe for children at appropriate dosages and with the same precautions as for adults (see below). The Tolerable Upper Intake Levels (ULs) set by the Institute of Medicine (IOM) in the USA, for children are based on age:2
• Birth−6 months: 4 mg per day
• 7−12 months: 5 mg per day
• 1−3 years: 7 mg per day
• 4−8 years: 12 mg per day
• 9−13 years: 23 mg per day
• 14−18 years: 34 mg per day
Safety
The main concern with longer-term excessive zinc intake is that it can reduce copper absorption and thus induce copper deficiency, which may lead to neurological disorders.7 The European Food Safety Authority (EFSA) and the UK Expert Group on Vitamins and Minerals (EVM) set a UL/Safe Upper Level of 25 mg per day for adults from supplements,7,188 whilst the IOM set a UL of 40 mg per day for adults on low-phytate diets.2
The only other side-effects noted in the British National Formulary (BNF) for zinc sulphate and acetate are digestive complaints: diarrhoea, gastritis, GI discomfort, nausea and vomiting.189
Caution
Caution is advised in people with renal impairment as zinc accumulation may occur.189
Interactions189
Calcium: Calcium may reduce the absorption of zinc.
Copper: Zinc hinders the absorption of copper.
Iron: Iron may reduce the absorption of zinc and vice versa.
Tetracycline and quinolone antibiotics: Zinc may reduce absorption of tetracycline and quinolone.
Penicillamine and trientine (chelating agents commonly used in Wilson’s disease): Zinc may reduce the absorption of penicillamine and trientine, and vice versa.
Pregnancy/lactation
Zinc needs are increased during pregnancy and lactation, and the same ULs as mentioned above apply during pregnancy and breastfeeding.7
In 2021, a Cochrane review of 25 RCTs, involving more than 18 000 pregnant women and their babies, reported no benefits for pregnancy outcomes with zinc supplementation, but also did not report any adverse effects, suggesting that zinc supplementation during pregnancy is safe.114
The BNF considers the risk in pregnancy and during breastfeeding: “theoretically minimal, but no information available”.189
Conclusion
Clinical trials have shown zinc supplementation to be safe and beneficial for a wide range of conditions. It should be noted that many of the studies were carried out in countries that have a high prevalence of zinc deficiency, and benefits may be more pronounced in, or even limited to, individuals with low zinc levels at baseline.
Acknowledgements
Author contributions: K. Elgar carried out the literature review and formulated the manuscript.
Peer-reviewers and editors: the Nutritional Medicine Institute thanks the peer-reviewers and editors for their important contributions.
Funding: Open Access publication was supported by an unrestricted donation from Pure Encapsulations, Sudbury, MA, USA. No other funding or sponsorship has been received for this work.
Declaration of interest: K. Elgar has received consultancy fees from Pure Encapsulations, Sudbury, MA, USA. This article is the independent work of the author and Pure Encapsulations was not involved in the decision to publish this research.
References
1 Chasapis, C. T., Ntoupa, P.-S. A., Spiliopoulou, C. A. & Stefanidou, M. E. (2020) Recent aspects of the effects of zinc on human health. Arch. Toxicol., 94, 1443–1460.
2 Institute of Medicine (2001) Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. The National Academies Press. doi:10.17226/10026.
3 Mousavi, S. M. et al. (2020) Clinical effectiveness of zinc supplementation on the biomarkers of oxidative stress: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res., 161, 105 166.
4 Prasad, A. S. (2014) Impact of the discovery of human zinc deficiency on health. J. Trace Elem. Med. Biol., 28, 357–363.
5 Wessells, K. R. & Brown, K. H. (2012) Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PLoS One, 7, e50568.
6 Committee on Medical Aspects of Food Policy (COMA) (1991) Dietary Reference Values for Food Energy and Nutrients for the United Kingdom (1991). https://www.gov.uk/government/publications/coma-reports.
7 European Food Safety Authority (EFSA) (2014) Scientific opinion on dietary reference values for zinc. EFSA J., 12, 3844.
8 Public Health England (2021) McCance and Widdowson’s composition of foods integrated dataset. Composition of Foods Integrated Dataset (CoFID). https://www.gov.uk/government/publications/composition-of-foods-integrated-dataset-cofid.
9 Wieringa, F. T., Dijkhuizen, M. A., Fiorentino, M., Laillou, A. & Berger, J. (2015) Determination of zinc status in humans: which indicator should we use? Nutrients, 7, 3252–3263.
10 Lowe, N. M., Fekete, K. & Decsi, T. (2009) Methods of assessment of zinc status in humans: a systematic review. Am. J. Clin. Nutr., 89, 2040S−2051S.
11 Bryce-Smith, D. & Hodgkinson, L. (1986) The Zinc Solution. Arrow Books.
12 Zdilla, M. J., Saling, J. R. & Starkey, L. D. (2016) Zinc sulfate taste acuity reflects dietary zinc intake in males. Clin. Nutr. ESPEN, 11, e21–e25.
13 Gruner, T. & Arthur, R. (2012) The accuracy of the Zinc Taste Test method. J. Altern. Complement. Med., 18, 541–550.
14 Faghfouri, A. H. et al. (2021) Clinical efficacy of zinc supplementation in improving antioxidant defense system: A comprehensive systematic review and time-response meta-analysis of controlled clinical trials. Eur. J. Pharmacol., 907, 174 243.
15 Hosseini, R., Ferns, G. A., Sahebkar, A., Mirshekar, M. A. & Jalali, M. (2021) Zinc supplementation is associated with a reduction in serum markers of inflammation and oxidative stress in adults: A systematic review and meta-analysis of randomized controlled trials. Cytokine, 138, 155 396.
16 Faghfouri, A. H. et al. (2021) Profiling inflammatory cytokines following zinc supplementation: a systematic review and meta-analysis of controlled trials. Br. J. Nutr., 126, 1441–1450. doi:10.1017/S0007114521000192.
17 Jayawardena, R., Ranasinghe, P., Kodithuwakku, W., Dalpatadu, C. & Attia, J. (2021) Zinc supplementation in pre-diabetes mellitus: a systematic review and meta-analysis. Minerva Endocrinol. doi:10.23736/S2724-6507.21.03234-X.
18 Jafari, A., Noormohammadi, Z., Askari, M. & Daneshzad, E. (2022) Zinc supplementation and immune factors in adults: a systematic review and meta-analysis of randomized clinical trials. Crit. Rev. Food Sci. Nutr., 62, 3023–3041. doi:10.1080/10408398.2020.1862048.
19 Mousavi, S. M., Djafarian, K., Mojtahed, A., Varkaneh, H. K. & Shab-Bidar, S. (2018) The effect of zinc supplementation on plasma C-reactive protein concentrations: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Pharmacol., 834, 10–16.
20 Yee, B. E., Richards, P., Sui, J. Y. & Marsch, A. F. (2020) Serum zinc levels and efficacy of zinc treatment in acne vulgaris: A systematic review and meta-analysis. Dermatol. Ther., 33, e14252.
21 Michaëlsson, G., Juhlin, L. & Vahlquist, A. (1977) Effects of oral zinc and vitamin A in acne. Arch. Dermatol., 113, 31–36.
22 Michaëlsson, G., Juhlin, L. & Ljunghall, K. (1977) A double-blind study of the effect of zinc and oxytetracycline in acne vulgaris. Br. J. Dermatol., 97, 561–566.
23 Weismann, K., Wadskov, S. & Sondergaard, J. (1977) Oral zinc sulphate therapy for acne vulgaris. Acta Derm. Venereol., 57, 357–360.
24 Cunliffe, W. J., Burke, B., Dodman, B. & Gould, D. J. (1979) A double-blind trial of a zinc sulphate/citrate complex and tetracycline in the treatment of acne vulgaris. Br. J. Dermatol., 101, 321–325.
25 Cunliffe, W. J. (1979) Unacceptable side-effects of oral zinc sulphate in the treatment of acne vulgaris. Br. J. Dermatol., 101, 363.
26 Tolino, E. et al. (2021) An open-label study comparing oral zinc to lymecycline in the treatment of acne vulgaris. J. Clin. Aesthet. Dermatol., 14, 56–58.
27 Chan, H., Chan, G., Santos, J., Dee, K. & Co, J. K. (2017) A randomized, double-blind, placebo-controlled trial to determine the efficacy and safety of lactoferrin with vitamin E and zinc as an oral therapy for mild to moderate acne vulgaris. Int. J. Dermatol., 56, 686–690.
28 Sardana, K. & Garg, V. K. (2010) An observational study of methionine-bound zinc with antioxidants for mild to moderate acne vulgaris. Dermatol. Ther., 23, 411–418.
29 Dreno, B., Amblard, P., Agache, P., Sirot, S. & Litoux, P. (1989) Low doses of zinc gluconate for inflammatory acne. Acta Derm. Venereol., 69, 541–543.
30 Dreno, B. et al. (2001) Multicenter randomized comparative double-blind controlled clinical trial of the safety and efficacy of zinc gluconate versus minocycline hydrochloride in the treatment of inflammatory acne vulgaris. Dermatology, 203, 135–140.
31 Luo, J., Mo, Y. & Liu, M. (2020) Blood and hair zinc levels in children with attention deficit hyperactivity disorder: A meta-analysis. Asian J. Psychiatr., 47, 101 805.
32 Ghoreishy, S. M., Ebrahimi Mousavi, S., Asoudeh, F. & Mohammadi, H. (2021) Zinc status in attention-deficit/hyperactivity disorder: a systematic review and meta-analysis of observational studies. Sci. Rep., 11, 14 612.
33 Noorazar, S. G., Malek, A., Aghaei, S. M., Yasamineh, N. & Kalejahi, P. (2020) The efficacy of zinc augmentation in children with attention deficit hyperactivity disorder under treatment with methylphenidate: A randomized controlled trial. Asian J. Psychiatr., 48, 101 868.
34 Salehi, B., Mohammadbeigi, A., Sheykholeslam, H., Moshiri, E. & Dorreh, F. (2016) Omega-3 and zinc supplementation as complementary therapies in children with attention-deficit/hyperactivity disorder. J. Res. Pharm. Pract., 5, 22–26.
35 Zamora, J. et al. (2011) [Zinc in the therapy of the attention-deficit/hyperactivity disorder in children. A preliminary randomized controlled trial]. Arch. Latinoam. Nutr., 61, 242–246.
36 Akhondzadeh, S., Mohammadi, M.-R. & Khademi, M. (2004) Zinc sulfate as an adjunct to methylphenidate for the treatment of attention deficit hyperactivity disorder in children: a double blind and randomized trial [ISRCTN64132371]. BMC Psychiatry, 4, 9.
37 Arnold, L. E. et al. (2011) Zinc for attention-deficit/hyperactivity disorder: placebo-controlled double-blind pilot trial alone and combined with amphetamine. J. Child. Adolesc. Psychopharmacol., 21, 1–19.
38 Bilici, M. et al. (2004) Double-blind, placebo-controlled study of zinc sulfate in the treatment of attention deficit hyperactivity disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry, 28, 181–190.
39 Lepping, P. & Huber, M. (2010) Role of zinc in the pathogenesis of attention-deficit hyperactivity disorder: implications for research and treatment. CNS Drugs, 24, 721–728.
40 Smailhodzic, D. et al. (2014) Zinc supplementation inhibits complement activation in age-related macular degeneration. PLoS One, 9, e112682.
41 Newsome, D. A. (2008) A randomized, prospective, placebo-controlled clinical trial of a novel zinc-monocysteine compound in age-related macular degeneration. Curr. Eye Res., 33, 591–598.
42 Vishwanathan, R., Chung, M. & Johnson, E. J. (2013) A systematic review on zinc for the prevention and treatment of age-related macular degeneration. Invest. Ophthalmol. Vis. Sci., 54, 3985–3998.
43 Newsome, D. A., Swartz, M., Leone, N. C., Elston, R. C. & Miller, E. (1988) Oral zinc in macular degeneration. Arch. Ophthalmol. (Chicago, Ill. 1960), 106, 192–198.
44 Jampol, L. M. & Ferris, F. L. 3rd (2001) Antioxidants and zinc to prevent progression of age-related macular degeneration. JAMA, 286, 2466–2468.
45 Stur, M., Tittl, M., Reitner, A. & Meisinger, V. (1996) Oral zinc and the second eye in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci., 37, 1225–1235.
46 Chen, M., Sun, Y. & Wu, Y. (2020) Lower circulating zinc and selenium levels are associated with an increased risk of asthma: evidence from a meta-analysis. Public Health Nutr., 23, 1555–1562.
47 Ghaffari, J., Alizadeh-Navaei, R., Dabaghzadeh, A. & Ghaffari, N. (2021) Serum zinc level and children`s asthma: A systematic and meta-analysis review article. Casp. J. Intern. Med., 12, 236–242.
48 Rerksuppaphol, S. & Rerksuppaphol, L. (2016) Zinc supplementation in children with asthma exacerbation. Pediatr. Rep., 8, 6685.
49 Ghaffari, J., Khalilian, A., Salehifar, E., Khorasani, E. & Rezaii, M. S. (2014) Effect of zinc supplementation in children with asthma: a randomized, placebo-controlled trial in northern Islamic Republic of Iran. East. Mediterr. Health J., 20, 391–396.
50 Zalewski, P. D. et al. (2005) Zinc metabolism in airway epithelium and airway inflammation: basic mechanisms and clinical targets. A review. Pharmacol. Ther., 105, 127–149.
51 Kim, J. E., Yoo, S. R., Jeong, M. G., Ko, J. Y. & Ro, Y. S. (2014) Hair zinc levels and the efficacy of oral zinc supplementation in patients with atopic dermatitis. Acta Derm. Venereol., 94, 558–562.
52 Ewing, C. I., Gibbs, A. C., Ashcroft, C. & David, T. J. (1991) Failure of oral zinc supplementation in atopic eczema. Eur. J. Clin. Nutr., 45, 507–510.
53 Ceylan, M. N., Akdas, S. & Yazihan, N. (2021) Is zinc an important trace element on bone-related diseases and complications? A meta-analysis and systematic review from serum level, dietary intake, and supplementation aspects. Biol. Trace Elem. Res., 199, 535–549.
54 Nakano, M., Nakamura, Y., Miyazaki, A. & Takahashi, J. (2021) Zinc pharmacotherapy for elderly osteoporotic patients with zinc deficiency in a clinical setting. Nutrients, 13, 1814.
55 Fung, E. B. et al. (2013) Zinc supplementation improves bone density in patients with thalassemia: a double-blind, randomized, placebo-controlled trial. Am. J. Clin. Nutr., 98, 960–971.
56 Peretz, A. et al. (2001) Zinc supplementation increases bone alkaline phosphatase in healthy men. J. Trace Elem. Med. Biol., 15, 175–178.
57 Sugiyama, T., Tanaka, H. & Kawai, S. (2000) Improvement of periarticular osteoporosis in postmenopausal women with rheumatoid arthritis by beta-alanyl-L-histidinato zinc: a pilot study. J. Bone Miner. Metab., 18, 335–338.
58 Clark, P. J., Eastell, R. & Barker, M. E. (1999) Zinc supplementation and bone growth in pubertal girls. Lancet (London, England), 354, 485.
59 Shiota, J., Tagawa, H., Izumi, N., Higashikawa, S. & Kasahara, H. (2015) Effect of zinc supplementation on bone formation in hemodialysis patients with normal or low turnover bone. Ren. Fail., 37, 57–60.
60 Pompano, L. M. & Boy, E. (2021) Effects of dose and duration of zinc interventions on risk factors for type 2 diabetes and cardiovascular disease: a systematic review and meta-analysis. Adv. Nutr., 12, 141–160.
61 Khazdouz, M. et al. (2020) Effects of zinc supplementation on cardiometabolic risk factors: a systematic review and meta-analysis of randomized controlled trials. Biol. Trace Elem. Res., 195, 373–398.
62 Ranasinghe, P. et al. (2015) Effects of zinc supplementation on serum lipids: a systematic review and meta-analysis. Nutr. Metab. (Lond.), 12, 26.
63 Li, Z., Wang, W., Liu, H., Li, S. & Zhang, D. (2019) The association of serum zinc and copper with hypertension: A meta-analysis. J. Trace Elem. Med. Biol., 53, 41–48.
64 Mousavi, S. M. et al. (2020) The effect of zinc supplementation on blood pressure: a systematic review and dose-response meta-analysis of randomized-controlled trials. Eur. J. Nutr., 59, 1815–1827.
65 Fernández-Cao, J. C. et al. (2018) Dietary zinc intake and whole blood zinc concentration in subjects with type 2 diabetes versus healthy subjects: A systematic review, meta-analysis and meta-regression. J. Trace Elem. Med. Biol., 49, 241–251.
66 Fernández-Cao, J. C. et al. (2019) Zinc intake and status and risk of type 2 diabetes mellitus: a systematic review and meta-analysis. Nutrients, 11, 1027.
67 Asbaghi, O. et al. (2020) Effects of zinc supplementation on lipid profile in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis., 30, 1260–1271.
68 Wang, X. et al. (2019) Zinc supplementation improves glycemic control for diabetes prevention and management: a systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr., 110, 76–90.
69 Jafarnejad, S., Mahboobi, S., McFarland, L. V., Taghizadeh, M. & Rahimi, F. (2019) Meta-analysis: effects of zinc supplementation alone or with multi-nutrients, on glucose control and lipid levels in patients with type 2 diabetes. Prev. Nutr. Food Sci., 24, 8–23.
70 Kelishadi, R. et al. (2010) Effect of zinc supplementation on markers of insulin resistance, oxidative stress, and inflammation among prepubescent children with metabolic syndrome. Metab. Syndr. Relat. Disord., 8, 505–510.
71 Hashemipour, M. et al. (2009) Effect of zinc supplementation on insulin resistance and components of the metabolic syndrome in prepubertal obese children. Hormones (Athens), 8, 279–285.
72 Warthon-Medina, M. et al. (2015) Zinc intake, status and indices of cognitive function in adults and children: a systematic review and meta-analysis. Eur. J. Clin. Nutr., 69, 649–661.
73 Yaffe, K., Clemons, T. E., McBee, W. L. & Lindblad, A. S. (2004) Impact of antioxidants, zinc, and copper on cognition in the elderly: a randomized, controlled trial. Neurology, 63, 1705–1707.
74 Maylor, E. A. et al. (2006) Effects of zinc supplementation on cognitive function in healthy middle-aged and older adults: the ZENITH study. Br. J. Nutr., 96, 752–760.
75 Donig, A. & Hautzinger, M. (2021) Zinc as an adjunct to antidepressant medication: a meta-analysis with subgroup analysis for different levels of treatment response to antidepressants. Nutr. Neurosci., 1–11. doi:10.1080/1028415X.2021.1888205.
76 Yosaee, S. et al. (2022) Zinc in depression: from development to treatment: a comparative/dose response meta-analysis of observational studies and randomized controlled trials. Gen. Hosp. Psychiatry, 74, 110−117. doi:10.1016/j.genhosppsych.2020.08.001.
77 Li, Z., Li, B., Song, X. & Zhang, D. (2017) Dietary zinc and iron intake and risk of depression: A meta-analysis. Psychiatry Res., 251, 41–47.
78 da Silva, L. E. M. et al. (2021) Zinc supplementation combined with antidepressant drugs for treatment of patients with depression: a systematic review and meta-analysis. Nutr. Rev., 79, 1–12.
79 Zhao, J. et al. (2016) Zinc levels in seminal plasma and their correlation with male infertility: A systematic review and meta-analysis. Sci. Rep., 6, 22 386.
80 Taravati, A. & Tohidi, F. (2016) Association between seminal plasma zinc level and asthenozoospermia: a meta-analysis study. Andrologia, 48, 646–653.
81 Gavella, M. & Lipovac, V. (1998) In vitro effect of zinc on oxidative changes in human semen. Andrologia, 30, 317–323.
82 Yazdanpanah, K., Moghimi, N., Yousefinejad, V., Ghaderi, E. & Darvishi, N. (2009) Effect of zinc sulphate on peptic ulcer disease. Pak. J. Med. Sci., 25, 404–407.
83 Yazdanpanah, K. et al. (2016) Efficacy of zinc sulfate in peptic ulcer disease: a randomized double-blind clinical trial study. J. Clin. Diagn. Res., 10, OC11−OC15.
84 Viñas, L. (1993) Prospecto copinal 300 mg capsulas. Patient Information Leaflet. https://cima.aemps.es/cima/dochtml/p/56926/P_56926.html.
85 Jiménez, E., Bosch, F., Galmés, J. L. & Baños, J. E. (1992) Meta-analysis of efficacy of zinc acexamate in peptic ulcer. Digestion, 51, 18–26.
86 Frommer, D. J. (1975) The healing of gastric ulcers by zinc sulphate. Med. J. Aust., 2, 793–796.
87 de Moura, M. S. B. et al. (2020) Zinc gluconate supplementation impacts the clinical improvement in patients with ulcerative colitis. Biometals, 33, 15–27.
88 Ananthakrishnan, A. N. et al. (2015) Zinc intake and risk of Crohn’s disease and ulcerative colitis: a prospective cohort study. Int. J. Epidemiol., 44, 1995–2005.
89 Dronfield, M. W., Malone, J. D. & Langman, M. J. (1977) Zinc in ulcerative colitis: a therapeutic trial and report on plasma levels. Gut, 18, 33–36.
90 Sturniolo, G. C., Di Leo, V., Ferronato, A., D’Odorico, A. & D’Incà, R. (2001) Zinc supplementation tightens ‘leaky gut’ in Crohn’s disease. Inflamm. Bowel Dis., 7, 94–98.
91 Belluzzi, A. et al. (1994) Short report: zinc sulphate supplementation corrects abnormal erythrocyte membrane long-chain fatty acid composition in patients with Crohn’s disease. Aliment. Pharmacol. Ther., 8, 127–130.
92 Brignola, C. et al. (1993) Zinc supplementation restores plasma concentrations of zinc and thymulin in patients with Crohn’s disease. Aliment. Pharmacol. Ther., 7, 275–280.
93 Tan, H. K., Streeter, A., Cramp, M. E. & Dhanda, A. D. (2020) Effect of zinc treatment on clinical outcomes in patients with liver cirrhosis: A systematic review and meta-analysis. World J. Hepatol., 12, 389–398.
94 Diglio, D. C. et al. (2020) Role of zinc supplementation in the management of chronic liver diseases: A systematic review and meta-analysis. Ann. Hepatol., 19, 190–196.
95 Shen, Y.-C., Chang, Y.-H., Fang, C.-J. & Lin, Y.-S. (2019) Zinc supplementation in patients with cirrhosis and hepatic encephalopathy: a systematic review and meta-analysis. Nutr. J., 18, 34.
96 Fathi, M., Alavinejad, P., Haidari, Z. & Amani, R. (2020) The effects of zinc supplementation on metabolic profile and oxidative stress in overweight/obese patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled trial. J. Trace Elem. Med. Biol., 62, 126 635.
97 Fathi, M., Alavinejad, P., Haidari, Z. & Amani, R. (2020) The effect of zinc supplementation on steatosis severity and liver function enzymes in overweight/obese patients with mild to moderate non-alcoholic fatty liver following calorie-restricted diet: a double-blind, randomized placebo-controlled trial. Biol. Trace Elem. Res., 197, 394–404.
98 Hassan, A., Sada, K.-K., Ketheeswaran, S., Dubey, A. K. & Bhat, M. S. (2020) Role of zinc in mucosal health and disease: a review of physiological, biochemical, and molecular processes. Cureus, 12, e8197.
99 Mahmood, A. et al. (2007) Zinc carnosine, a health food supplement that stabilises small bowel integrity and stimulates gut repair processes. Gut, 56, 168–175.
100 Chaitanya, N. C. et al. (2019) A meta-analysis on the efficacy of zinc in oral mucositis during cancer chemo and/or radiotherapy-an evidence-based approach. J. Nutr. Sci. Vitaminol. (Tokyo), 65, 184–191.
101 Anandhi, P., Sharief, R. M. & Rahila, C. (2020) The benefit of zinc sulfate in oropharyngeal mucositis during hyperfractionated accelerated concomitant boost radiotherapy with concurrent cisplatin for advanced-stage oropharyngeal and hypopharyngeal cancers. Indian J. Palliat. Care, 26, 437–443.
102 Gutiérrez-Vargas, R., Villasis-Keever, M.-Á., Portilla-Robertson, J., Ascencio-Montiel, I.-D. & Zapata-Tarrés, M. (2020) Effect of zinc on oropharyngeal mucositis in children with acute leukemia undergoing chemotherapy. Med. Oral Patol. Oral Cir. Bucal, 25, e791–e798.
103 Funato, M. et al. (2018) Prophylactic effect of polaprezinc, a zinc-L-carnosine, against chemotherapy-induced oral mucositis in pediatric patients undergoing autologous stem cell transplantation. Anticancer Res., 38, 4691–4697.
104 Kim, J. et al. (2015) Anti-inflammatory effects of zinc in PMA-treated human gingival fibroblast cells. Med. Oral Patol. Oral Cir. Bucal, 20, e180−e187.
105 Al-Maweri, S. A. et al. (2021) Association between serum zinc levels and recurrent aphthous stomatitis: a meta-analysis with trial sequential analysis. Clin. Oral Investig., 25, 407–415.
106 Sharquie, K. E., Najim, R. A., Al-Hayani, R. K., Al-Nuaimy, A. A. & Maroof, D. M. (2008) The therapeutic and prophylactic role of oral zinc sulfate in management of recurrent aphthous stomatitis (ras) in comparison with dapsone. Saudi Med. J., 29, 734–738.
107 Orbak, R., Cicek, Y., Tezel, A. & Dogru, Y. (2003) Effects of zinc treatment in patients with recurrent aphthous stomatitis. Dent. Mater. J., 22, 21–29.
108 Ghorbani, A., Akbari, J., Boorboor, M., Nekoukar, Z. & Eslami, G. (2020) Evaluation of zinc sulfate mucoadhesive formulation on recurrent aphthous stomatitis: a randomized double-blind, placebo-controlled clinical trial. BMC Oral Health, 20, 212.
109 Merchant, H. W., Gangarosa, L. P., Glassman, A. B. & Sobel, R. E. (1977) Zinc sulfate supplementation for treatment of recurring oral ulcers. South. Med. J., 70, 559–561.
110 Harding, M. (2016) Polycystic ovary syndrome. patient.info https://patient.info/doctor/polycystic-ovary-syndrome-pro.
111 Foroozanfard, F. et al. (2015) Effects of zinc supplementation on markers of insulin resistance and lipid profiles in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Exp. Clin. Endocrinol. Diabetes, 123, 215–220.
112 Jamilian, M. et al. (2016) Effects of zinc supplementation on endocrine outcomes in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Biol. Trace Elem. Res., 170, 271–278.
113 Jamilian, M., Maktabi, M. & Asemi, Z. (2017) A trial on the effects of magnesium-zinc-calcium-vitamin D co-supplementation on glycemic control and markers of cardio-metabolic risk in women with polycystic ovary syndrome. Arch. Iran. Med., 20, 640–645.
114 Carducci, B., Keats, E. C. & Bhutta, Z. A. (2021) Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst. Rev., 3, CD000230.
115 Karamali, M. et al. (2016) Zinc supplementation and the effects on pregnancy outcomes in gestational diabetes: a randomized, double-blind, placebo-controlled trial. Exp. Clin. Endocrinol. Diabetes, 124, 28–33.
116 Karamali, M. et al. (2015) Zinc supplementation and the effects on metabolic status in gestational diabetes: A randomized, double-blind, placebo-controlled trial. J. Diabetes Complications, 29, 1314–1319.
117 Jamilian, M. et al. (2019) The effects of magnesium-zinc-calcium-vitamin D co-supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. BMC Pregnancy Childbirth, 19, 107.
118 Bramley, T. J., Lerner, D. & Sames, M. (2002) Productivity losses related to the common cold. J. Occup. Environ. Med., 44, 822–829.
119 Hemilä, H., Petrus, E. J., Fitzgerald, J. T. & Prasad, A. (2016) Zinc acetate lozenges for treating the common cold: an individual patient data meta-analysis. Br. J. Clin. Pharmacol., 82, 1393–1398.
120 Hemilä, H., Fitzgerald, J. T., Petrus, E. J. & Prasad, A. (2017) Zinc acetate lozenges may improve the recovery rate of common cold patients: an individual patient data meta-analysis. Open Forum Infect. Dis., 4, ofx059.
121 Hemilä, H. (2017) Zinc lozenges and the common cold: a meta-analysis comparing zinc acetate and zinc gluconate, and the role of zinc dosage. JRSM Open, 8, 2054270417694291.
122 Farr, B. M. & Gwaltney, J. M. J. (1987) The problems of taste in placebo matching: an evaluation of zinc gluconate for the common cold. J. Chronic Dis., 40, 875–879.
123 Hemilä, H., Haukka, J., Alho, M., Vahtera, J. & Kivimäki, M. (2020) Zinc acetate lozenges for the treatment of the common cold: a randomised controlled trial. BMJ Open, 10, e031662.
124 Rerksuppaphol, S. & Rerksuppaphol, L. (2013) A randomized controlled trial of chelated zinc for prevention of the common cold in Thai school children. Paediatr. Int. Child Health, 33, 145–150.
125 Kurugöl, Z., Akilli, M., Bayram, N. & Koturoglu, G. (2006) The prophylactic and therapeutic effectiveness of zinc sulphate on common cold in children. Acta Paediatr., 95, 1175–1181.
126 McElroy, B. H. & Miller, S. P. (2003) An open-label, single-center, phase IV clinical study of the effectiveness of zinc gluconate glycine lozenges (Cold-Eeze) in reducing the duration and symptoms of the common cold in school-aged subjects. Am. J. Ther., 10, 324–329.
127 McElroy, B. H. & Miller, S. P. (2002) Effectiveness of zinc gluconate glycine lozenges (Cold-Eeze) against the common cold in school-aged subjects: a retrospective chart review. Am. J. Ther., 9, 472–475.
128 Kurugöl, Z., Bayram, N. & Atik, T. (2007) Effect of zinc sulfate on common cold in children: randomized, double blind study. Pediatr. Int., 49, 842–847.
129 Macknin, M. L., Piedmonte, M., Calendine, C., Janosky, J. & Wald, E. (1998) Zinc gluconate lozenges for treating the common cold in children: a randomized controlled trial. JAMA, 279, 1962–1967.
130 Korant, B. D. & Butterworth, B. E. (1976) Inhibition by zinc of rhinovirus protein cleavage: interaction of zinc with capsid polypeptides. J. Virol., 18, 298–306.
131 Jacobs, S. E., Lamson, D. M., St George, K. & Walsh, T. J. (2013) Human rhinoviruses. Clin. Microbiol. Rev., 26, 135–162.
132 World Health Organization (2019) Pneumonia. https://www.who.int/news-room/fact-sheets/detail/pneumonia.
133 Brown, N., Kukka, A. J. & Mårtensson, A. (2020) Efficacy of zinc as adjunctive pneumonia treatment in children aged 2 to 60 months in low-income and middle-income countries: a systematic review and meta-analysis. BMJ Paediatrics Open, 4, e000662.
134 Wang, L. & Song, Y. (2018) Efficacy of zinc given as an adjunct to the treatment of severe pneumonia: A meta-analysis of randomized, double-blind and placebo-controlled trials. Clin. Respir. J., 12, 857–864.
135 Tie, H.-T. et al. (2016) Zinc as an adjunct to antibiotics for the treatment of severe pneumonia in children <5 years: a meta-analysis of randomised-controlled trials. Br. J. Nutr., 115, 807–816.
136 Lassi, Z. S., Moin, A. & Bhutta, Z. A. (2016) Zinc supplementation for the prevention of pneumonia in children aged 2 months to 59 months. Cochrane Database Syst. Rev., 12, CD005978.
137 Cherasse, Y. & Urade, Y. (2017) Dietary zinc acts as a sleep modulator. Int. J. Mol. Sci., 18, 2334.
138 Saito, H. et al. (2017) Zinc-rich oysters as well as zinc-yeast- and astaxanthin-enriched food improved sleep efficiency and sleep onset in a randomized controlled trial of healthy individuals. Mol. Nutr. Food Res., 61. doi: 10.1002/mnfr.201600882.
139 Gholipour Baradari, A. et al. (2018) The effect of zinc supplementation on sleep quality of ICU nurses: a double blinded randomized controlled trial. Workplace Health Saf., 66, 191–200.
140 Jafari, F., Tarrahi, M. J., Farhang, A. & Amani, R. (2020) Effect of zinc supplementation on quality of life and sleep quality in young women with premenstrual syndrome: a randomized, double-blind, placebo-controlled trial. Arch. Gynecol. Obstet., 302, 657–664.
141 Maxfield, L., Shukla, S. & Crane, J. S. (2021) Zinc deficiency.
142 Munankarmi, D. (2017) Management of dysgeusia related to cancer. J. Lumbini Med. Coll., 5, 3−12.
143 Chi, W. J. et al. (2020) The effects of zinc on radiation-induced dysgeusia: a systematic review and meta-analysis. Support. Care Cancer, 28, 1–12.
144 Khan, A. H., Safdar, J. & Siddiqui, S. U. (2019) Efficacy of zinc sulfate on concurrent chemoradiotherapy induced taste alterations in oral cancer patients: A double blind randomized controlled trial. Pakistan J. Med. Sci., 35, 624–629.
145 Lyckholm, L. et al. (2012) A randomized, placebo controlled trial of oral zinc for chemotherapy-related taste and smell disorders. J. Pain Palliat. Care Pharmacother., 26, 111–114.
146 Fujii, H. et al. (2018) Improvement of dysgeusia by polaprezinc, a zinc-L-carnosine, in outpatients receiving cancer chemotherapy. Anticancer Res., 38, 6367–6373.
147 Matson, A. et al. (2003) Zinc supplementation at conventional doses does not improve the disturbance of taste perception in hemodialysis patients. J. Ren. Nutr., 13, 224–228.
148 Watson, A. R., Stuart, A., Wells, F. E., Houston, I. B. & Addison, G. M. (1983) Zinc supplementation and its effect on taste acuity in children with chronic renal failure. Hum. Nutr. Clin. Nutr., 37, 219–225.
149 Greger, J. L. & Geissler, A. H. (1978) Effect of zinc supplementation on taste acuity of the aged. Am. J. Clin. Nutr., 31, 633–637.
150 Stewart-Knox, B. J. et al. (2008) Taste acuity in response to zinc supplementation in older Europeans. Br. J. Nutr., 99, 129–136.
151 Ikeda, M. et al. (2013) [The effect of zinc agent in 219 patients with zinc deficiency-inductive/ idiopathic taste disorder: a placebo controlled randomized study]. Nihon Jibiinkoka Gakkai Kaiho, 116, 17–26.
152 Takaoka, T. et al. (2010) Effects of zinc supplementation on serum zinc concentration and ratio of apo/holo-activities of angiotensin converting enzyme in patients with taste impairment. Auris. Nasus. Larynx, 37, 190–194.
153 Sakagami, M. et al. (2009) A zinc-containing compound, Polaprezinc, is effective for patients with taste disorders: randomized, double-blind, placebo-controlled, multi-center study. Acta Otolaryngol., 129, 1115–1120.
154 Heckmann, S. M. et al. (2005) Zinc gluconate in the treatment of dysgeusia−a randomized clinical trial. J. Dent. Res., 84, 35–38.
155 Yoshida, S., Endo, S. & Tomita, H. (1991) A double-blind study of the therapeutic efficacy of zinc gluconate on taste disorder. Auris. Nasus. Larynx, 18, 153–161.
156 Tupe, R. P. & Chiplonkar, S. A. (2009) Zinc supplementation improved cognitive performance and taste acuity in Indian adolescent girls. J. Am. Coll. Nutr., 28, 388–396.
157 Sakai, F., Yoshida, S., Endo, S. & Tomita, H. (2002) Double-blind, placebo-controlled trial of zinc picolinate for taste disorders. Acta Otolaryngol. Suppl., 129–133. doi:10.1080/00016480260046517.
158 Henkin, R. I., Schecter, P. J., Friedewald, W. T., Demets, D. L. & Raff, M. (1976) A double blind study of the effects of zinc sulfate on taste and smell dysfunction. Am. J. Med. Sci., 272, 285–299.
159 Henkin, R. I., Martin, B. M. & Agarwal, R. P. (1999) Efficacy of exogenous oral zinc in treatment of patients with carbonic anhydrase VI deficiency. Am. J. Med. Sci., 318, 392–405.
160 Mahmoodianfard, S. et al. (2015) Effects of zinc and selenium supplementation on thyroid function in overweight and obese hypothyroid female patients: a randomized double-blind controlled trial. J. Am. Coll. Nutr., 34, 391–399.
161 Miyamoto, T., Sakurai, A. & Degroot, L. J. (1991) Effects of zinc and other divalent metals on deoxyribonucleic acid binding and hormone-binding activity of human al thyroid hormone receptor expressed in Escherichia coli*. Endocrinology, 129, 3027–3033.
162 Pekary, A. E., Lukaski, H. C., Mena, I. & Hershman, J. M. (1991) Processing of TRH precursor peptides in rat brain and pituitary is zinc dependent. Peptides, 12, 1025–1032.
163 Beserra, J. B. et al. (2021) Relation between zinc and thyroid hormones in humans: a systematic review. Biol. Trace Elem. Res., 199, 4092−4100. doi:10.1007/s12011-020-02562-5.
164 Rabbani, E. et al. (2021) Randomized study of the effects of zinc, vitamin A, and magnesium co-supplementation on thyroid function, oxidative stress, and hs-CRP in patients with hypothyroidism. Biol. Trace Elem. Res., 199, 4074−4083. doi:10.1007/s12011-020-02548-3.
165 Nakamura, H. et al. (2019) Zinc deficiency exacerbates pressure ulcers by increasing oxidative stress and ATP in the skin. J. Dermatol. Sci., 95, 62–69.
166 Miletta, M. C., Schöni, M. H., Kernland, K., Mullis, P. E. & Petkovic, V. (2013) The role of zinc dynamics in growth hormone secretion. Horm. Res. Paediatr., 80, 381–389.
167 Houston, S., Haggard, J., Williford, J. J., Meserve, L. & Shewokis, P. (2001) Adverse effects of large-dose zinc supplementation in an institutionalized older population with pressure ulcers. J. Am. Geriatr. Soc., 49, 1130–1132.
168 Moore, Z. et al. (2019) The prevalence of pressure ulcers in Europe, what does the European data tell us: a systematic review. J. Wound Care, 28, 710–719.
169 Song, Y.-P. et al. (2020) Zinc therapy is a reasonable choice for patients with pressure injuries: a systematic review and meta-analysis. Nutr. Clin. Pract., 35, 1001–1009.
170 Sakae, K. & Yanagisawa, H. (2014) Oral treatment of pressure ulcers with polaprezinc (zinc L-carnosine complex): 8-week open-label trial. Biol. Trace Elem. Res., 158, 280–288.
171 Desneves, K. J., Todorovic, B. E., Cassar, A. & Crowe, T. C. (2005) Treatment with supplementary arginine, vitamin C and zinc in patients with pressure ulcers: a randomised controlled trial. Clin. Nutr., 24, 979–987.
172 Kohn, S., Kohn, D. & Schiller, D. (2000) Effect of zinc supplementation on epidermal Langerhans’ cells of elderly patients with decubital ulcers. J. Dermatol., 27, 258–263.
173 Greaves, M. W. & Skillen, A. W. (1970) Effects of long-continued ingestion of zinc sulphate in patients with venous leg ulceration. Lancet (London, England), 2, 889–891.
174 Myers, M. B. & Cherry, G. (1970) Zinc and the healing of chronic leg ulcers. Am. J. Surg., 120, 77–81.
175 Greaves, M. W. & Ive, F. A. (1972) Double-blind trial of zinc sulphate in the treatment of chronic venous leg ulceration. Br. J. Dermatol., 87, 632–634.
176 Phillips, A., Davidson, M. & Greaves, M. W. (1977) Venous leg ulceration: evaluation of zinc treatment, serum zinc and rate of healing. Clin. Exp. Dermatol., 2, 395–399.
177 Floersheim, G. L. & Lais, E. (1980) [Lack of effect of oral zinc sulfate on wound healing in leg ulcer]. Schweiz. Med. Wochenschr., 110, 1138–1145.
178 Momen-Heravi, M. et al. (2017) The effects of zinc supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. Wound Repair Regen., 25, 512–520.
179 Petry, N., Olofin, I., Boy, E., Donahue Angel, M. & Rohner, F. (2016) The effect of low dose iron and zinc intake on child micronutrient status and development during the first 1000 days of life: a systematic review and meta-analysis. Nutrients, 8, 773.
180 Gera, T., Shah, D. & Sachdev, H. S. (2019) Zinc supplementation for promoting growth in children under 5 years of age in low- and middle-income countries: a systematic review. Indian Pediatr., 56, 391–406.
181 Nissensohn, M. et al. (2016) Effect of zinc intake on growth in infants: a meta-analysis. Crit. Rev. Food Sci. Nutr., 56, 350–363.
182 World Health Organization (2021) Malnutrition. Factsheets. https://www.who.int/news-room/fact-sheets/detail/malnutrition.
183 Sajedi, F., Shahshahani, S., Ghiasvand, H., Mosallanezhad, Z. & Fatollahierad, S. (2020) Does zinc with and without iron co-supplementation have effect on motor and mental development of children? A systematic review and meta-analysis. BMC Pediatr., 20, 451.
184 Gogia, S. & Sachdev, H. S. (2012) Zinc supplementation for mental and motor development in children. Cochrane Database Syst. Rev., 12, CD007991.
185 Khan, W. U. & Sellen, D. W. (2011) Zinc supplementation in the management of diarrhoea: biological, behavioural and contextual rationale. WHO e-Library of Evidence for Nutrition Actions. https://www.who.int/elena/titles/bbc/zinc_diarrhoea/en/.
186 Lazzerini, M. & Wanzira, H. (2016) Oral zinc for treating diarrhoea in children. Cochrane Database Syst. Rev., 12, CD005436.
187 Rouhani, P., Rezaei Kelishadi, M. & Saneei, P. (2022) Effect of zinc supplementation on mortality in under 5-year children: a systematic review and meta-analysis of randomized clinical trials. Eur. J. Nutr., 61, 37−54. doi:10.1007/s00394-021-02604-1.
188 Expert Group on Vitamins and Minerals (2003) Safe Upper Levels for Vitamins and Minerals. https://webarchive.nationalarchives.gov.uk/ukgwa/20200803163143/https://cot.food.gov.uk/cotreports/cotjointreps/evmreport.
189 National Institute for Health and Care Excellence (NICE). Zinc sulfate. British National Formula (BNF). https://bnf.nice.org.uk/drug/zinc-sulfate.html#indicationsAndDoses.