By Karin Elgar
Abstract
Introduction
General effects and mechanisms of action of Vitamin D
Clinical uses
Safety
Conclusion
Acknowledgements
References
Abstract
Vitamin D3 is produced in the skin on exposure to ultraviolet B radiation, and is metabolised in the liver and kidneys to the biologically active form of vitamin D that binds to vitamin D receptors. Vitamin D was first recognised for its importance in calcium metabolism and therefore bone health, with the classic deficiency disease being rickets in children and osteomalacia in adults, but is now also known for its importance in modulating immunity.
Epidemiological studies have linked vitamin D deficiency to many conditions, including heart disease, cancer, allergies and autoimmunity. Vitamin D supplementation trials have confirmed benefits for some conditions, including atopic dermatitis, chronic urticaria, colorectal cancer, depression, polycystic ovary syndrome and type 2 diabetes mellitus, but not others, such as multiple sclerosis, prevention of allergic sensitisation in infants and psoriasis. Whilst there is some evidence of benefits for cardiovascular risk factors, this does not translate to a reduction in cardiovascular events in clinical trials.
Vitamin D is generally considered safe, and the upper limit set by the National Institute of Health is 4000 IU per day. The main safety concern with vitamin D is hypercalcaemia, based on its role in calcium metabolism, and caution is therefore advised in conditions and with medications that can also affect calcium metabolism.
Cite as: Elgar, K. (2022) Vitamin D: A Review of Clinical Use and Efficacy. Nutr Med J., 1 (2): 54-80.
Affiliation: K. Elgar is with the Nutritional Medicine Institute, London, UK.
Article history: Received 02 May 2021; Peer-reviewed and received in revised form 19 August 2021; Accepted 02 September 2021. Available online 4 July 2022.
Published by: The Nutritional Medicine Institute
Open Access: This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs licence (http:// creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reproduction and distribution of the work, in any medium, provided the original work is not altered or transformed in any way, and that the work is properly cited. For commercial use please contact support@nmi.health
Introduction
Vitamin D3, or cholecalciferol, is the natural form of vitamin D that is produced in the skin on exposure to ultraviolet B radiation and is also found in some foods, in particular oily fish.1 Other food sources include eggs and milk products, but levels in these foods are low. Vitamin D2, or ergocalciferol, is of plant or fungal origin.2 Although we can get vitamin D through our diet, it is strictly speaking not a vitamin but a steroid hormone.3 Vitamin D3 is metabolised in the liver to 25-hydroxyvitamin D3, also called calcifediol or calcidiol, which is converted further in the kidneys to 1α,25-dihydroxyvitamin D3, the biologically active form of vitamin D that binds to vitamin D receptors (VDRs).4
Vitamin D was first recognised for its role in calcium and phosphorous metabolism, and thus playing an important role in bone mineralisation, with the classic vitamin D deficiency (VDD) diseases being rickets in children and osteomalacia in adults. Over the past decades, however, it has become clear that VDRs are expressed in almost all cells,2 and that vitamin D has many important physiological roles, in particular in terms of modulating the immune system.5
Michael Holick, a well-known expert on vitamin D, defines VDD as a vitamin D level of below 20 ng/ml (50 nmol/l),a insufficiency as 20−29.9 ng/ml (50−74.9 nmol/l) and sufficiency as 30 ng/ml (75 nmol/l) or more, with levels of 150 ng/ml or higher potentially toxic (Table 1).1 These cut-off values are commonly used in vitamin D research and, unless otherwise noted, have been used in the research quoted in this paper. Some authorities, however, set lower cut-off points, for example the National Institute of Health (NIH) in the USA considers levels of 20 ng/ml and higher as adequate for healthy people, and a level of over 50 ng/ml as potentially harmful,6 whilst the National Institute for Health and Care Excellence (NICE) in the UK defines VDD as a level of less than 10 ng/ml.7
Table 1: Serum 25(OH)D reference ranges and lab equivalents210
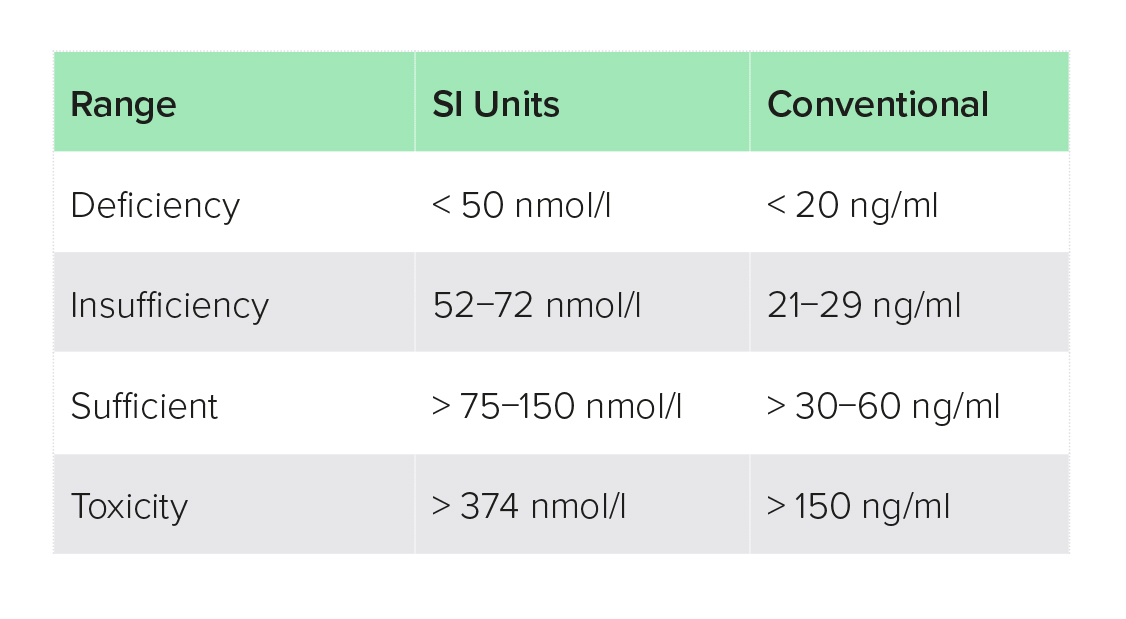 Table 1. Recommended ranges vary. In the UK, NICE recommends > 25 ng/ml, while others including The Endocrine Society, National Osteoporosis Foundation, International Osteoporosis Foundation, American Association for Clinical Endocrinologists, and the American Geriatric Society recommend > 30 ng/ml.
Table 1. Recommended ranges vary. In the UK, NICE recommends > 25 ng/ml, while others including The Endocrine Society, National Osteoporosis Foundation, International Osteoporosis Foundation, American Association for Clinical Endocrinologists, and the American Geriatric Society recommend > 30 ng/ml.
Epidemiological studies have linked VDD to many conditions, including heart disease, cancer, allergies and autoimmunity,5 but evidence from vitamin D supplementation trials is contradictory and does not confirm a protective effect for various conditions.8 Whilst epidemiological studies have established associations between vitamin D levels and disease, they cannot prove cause and effect. Vitamin D levels may be a proxy for other factors, such as exposure to sunshine, which has also been shown to exert other biological effects, including nitric oxide, serotonin and melatonin production and regulating circadian rhythm.9,10
Many intervention studies have therefore tried to establish whether vitamin D supplementation can help prevent or treat various conditions, which will be discussed in the section ‘Clinical Uses’. For many conditions, results have been contradictory. Such inconsistencies may be explained by a number of limitations with regards to study designs.
Limitations in clinical trials on vitamin D supplementation
Some, but not all, studies have established vitamin D levels at baseline and after supplementation. Without this information it is difficult to know whether or not supplementation was effective in raising vitamin D levels appropriately.
Even where this was done, measuring vitamin D levels to establish deficiency or sufficiency has been shown to be fraught with problems.4 The activated forms of the vitamins have short half-lives (4−6 hours) and concentrations in blood are very low, making them difficult to measure. 25(OH)D2/3 have much longer half-lives (25 days) and are therefore usually measured, although there is great variability in results, which is to some extent based on different methodologies being used and also due to the fact that vitamin D is bound to carrier proteins in blood.4
Vitamin D dosing regimens have also varied widely from daily dosages of 200 IU to bolus doses of up to 500 000 IU every 3 months or just once. Most studies have used oral vitamin D3, but some have used vitamin D2, active metabolites or analogues, either orally or in injectable forms. Unless discussed specifically under the respective condition, there was no clear trend as to whether certain dosing regimens were superior to others. Also, unless otherwise noted, throughout this paper the term ‘vitamin D’ refers to vitamin D3.
Finally, patient populations have varied and there are known differences amongst certain patient groups, for example, it has been shown that obese people tend to respond less well to supplementation.11 There are also significant inter-individual differences in response to vitamin D supplementation and vitamin D metabolism.5 There is a growing body of evidence that certain genetic single-nucleotide polymorphisms (SNPs; spontaneous alterations of particular genes) are involved in vitamin D metabolism, leading to a much higher requirement for people with specific SNPs to see benefits.5 The discussion of this topic is outside the scope of this white paper.
The aim of this paper is to review the evidence from clinical intervention trials on the effectiveness of vitamin D in either preventing or reversing conditions commonly associated with vitamin D.
General effects and mechanisms of action of vitamin D
Vitamin D exerts its effects mostly on the genetic level in that it binds to a VDR, which is a transcription factor. That is, on activation through vitamin D, it binds to specific sites in the DNA, regulating the expression of hundreds of genes in a tissue/cell-specific fashion.2
Calcium metabolism
Together with parathyroid hormone (PTH), vitamin D is essential for the maintenance of serum calcium concentrations that have to be within a narrow range for normal cellular function, in particular of the nervous system. When serum calcium levels fall, vitamin D triggers the expression of genes that increase absorption of calcium in the intestine and reabsorption through the kidneys as well as releasing calcium from the bones when dietary intake is low.12 In a similar way, vitamin D and PTH are also involved in phosphorous metabolism.13 As such, vitamin D plays an important role in bone mineralisation and with that in bone health.
Immune modulation
Vitamin D receptors are expressed on almost all immune cells, and vitamin D is essential for the proper functioning of both the innate and the adaptive immune systems. Whilst it promotes innate immunity, the first line of defence against pathogens that is non-specific, it appears to have a more inhibitory or regulatory effect on the adaptive, acquired immune system, mostly through regulating gene expression.2 Some evidence also suggests that vitamin D may modulate immunity directly, i.e. without regulating gene expression, through stabilising endothelial membranes.5
Vitamin D appears to have an immune-regulatory effect by moving the immune system from a pro-inflammatory to an anti-inflammatory milieu.14 It shows benefit in infectious conditions as well as in conditions with excessive inflammation, such as allergies and autoimmunity.
Clinical uses
Allergy/atopy
Due to its known immune modulatory properties and epidemiological research showing that vitamin D levels are lower in patients with allergic conditions, vitamin D supplementation trials have looked into its effects on both prevention and treatment of allergic and atopic conditions.
Prevention of allergic sensitisation in infants
Two trials investigating vitamin D supplementation of infants in the first year of life found no protective effect on the development of allergies, with either 400 IU or 1200 IU vitamin D per day.15,16 In fact, infants in the high-dose supplementation group had a higher risk of milk allergy compared with the low-dose group, and high vitamin D levels in cord blood at birth was associated with an increased risk of allergic sensitisation at 12 months.16
A review from 2018 also found no evidence for beneficial effects of vitamin D supplementation for either the pregnant or breastfeeding mother or the infant, although the number and quality of studies reported here were considered to be low.17
Allergic rhinitis
There appears to be an inverse relationship between vitamin D levels and allergic rhinitis, although study results are inconsistent.18,19
One vitamin D supplementation randomised-controlled trial (RCT) found benefits in terms of a significantly reduced allergic rhinitis symptom score in adults with VDD at baseline with a dose of 50 000 IU per week for 8 weeks alongside the antihistamine cetirizine.20 Another RCT found benefits of reduced symptoms and medication use in children aged 5−12 years with allergic rhinitis with 1000 IU per day for 5 months.21 Interestingly, in this study none of the children was VDD at baseline, and vitamin D levels at the end of study were similar in both the vitamin D and placebo groups. However, there was a much greater increase in vitamin D level from a lower baseline in the vitamin D group.
Whilst evidence is limited, vitamin D alongside standard treatment appears to have additional benefits for adult and paediatric patients with allergic rhinitis. The best levels of supplementation may depend on whether or not patients are VDD
at the outset.
Asthma
Asthma is a chronic inflammatory condition of the airways characterised by symptoms of attacks of shortness of breath, chest tightness, wheezing and coughing.22 There is evidence for a link between asthma and vitamin D from both epidemiological and animal research, which is thought to be mediated through its effect on innate and adaptive immunity.23
A Cochrane review in 2016 pooled data from nine double-blind RCTs, seven of those in children and two in adults, and found significant reductions in exacerbations requiring either systemic corticosteroids, visits to the emergency department or hospitalisation.24 No effects were seen on forced expiratory volume in 1 second (FEV1; a measure of respiratory function) or Asthma Control Test scores. The evidence was judged to be of high quality. Several more meta-analyses, using largely the same set of trials, have come to the same conclusions.23,25,26
Effects appear to be limited to patients with low levels of vitamin D (< 25 nmol/l) at baseline.25,26 Dosing regimens, on the other hand, did not appear to affect results, although they have varied widely, from 600 IU daily to 60 000 IU monthly, or combinations of a large bolus dose followed by low-dose daily supplementation.23,25,26
Since these meta-analyses were done, a number of additional RCTs have been published with inconsistent results. Two studies in children failed to show significant improvements in number of exacerbations, although numbers of children with exacerbations were small in both studies, which may have affected statistical power,27,28 whilst another trial in children was terminated early due to futility.29 One study in children compared a bolus vitamin D2 injection followed by low-dose (400 IU vitamin D3 per day) oral supplement with oral supplementation of 400 IU vitamin D3 per day alone, and found that the bolus improved outcomes at 3 months more in severely deficient children, but there were no long-term differences in outcomes.30 Another study, looking at dosing regimens in asthmatic children, found that oral bolus supplementation with 100 000 IU once in fall and once in winter only achieved sufficiency in just over 50% of children with insufficient levels at baseline (none of the children was classed as deficient).31 Asthma-related outcomes were no different to the placebo group in this study.
On the other hand, two RCTs have found benefits in adults with asthma and vitamin D insufficiency/deficiency with regards to Asthma Control Test, quality of life, number of attacks and oral steroid use,32 and respiratory function and inflammatory markers,22 respectively. Patients received calcifediol 16 000 IU per week for 6 months and a single dose of vitamin D, 300 000 IU intramuscularly, respectively.
Overall, whilst evidence is inconsistent, there appears to be a benefit at least for those patients, both adults and children, who have insufficient vitamin D levels. A variety of dosing regimens appears to be beneficial.
Eczema/atopic dermatitis (AD)
Eczema or AD is an inflammatory condition of the skin that can cause itching, and severity of disease is associated with quality of life. It is thought to be due to a dysfunction of both the innate and adaptive immune systems, and issues with the barrier function of the skin.33 Observational studies have shown that low serum levels of vitamin D are associated with AD.34
Three meta-analyses including three to four clinical trials all came to the conclusion that vitamin D supplementation can reduce the severity of AD.33,34,35 Dosages used across the studies varied from 1000 IU per day to 6000 IU per day for up to 3 months.
Since then, three RCTs in children have been published that were not included in the meta-analyses. Two of them found significant improvements in severity scores of AD,36,37 whilst one found no significant improvement over placebo.38 Children in the latter study, however, had a mean vitamin D level of 47.1 nmol/l at baseline, with no difference in baseline vitamin D levels between the vitamin D and the placebo groups. The former two studies on the other hand included only children who were vitamin D deficient or insufficient. An RCT including children and adults with AD found that patients who had vitamin D levels of > 20 ng/ml had lower severity scores than those with lower vitamin D levels, whilst there was no difference between those who had levels of 20−30 ng/ml versus those with levels above 30 ng/ml.39 These findings were regardless of whether or not that vitamin D level was achieved through supplementation, although all of the participants receiving vitamin D had levels > 30 ng/ml, whilst none of the participants on placebo did, but 41% of the latter had levels of 20−30 ng/ml.
Overall, the evidence, mostly in children, suggests that vitamin D is of benefit to patients with AD, at least for those with VDD. Dosages ranged from 1000 to 6000 IU per day, and study duration was mostly 3 months.
Autoimmunity
The NIH in the USA estimates that up to 23.5 million Americans have autoimmune diseases, making it one of the most prevalent group of diseases in the USA.40 Worldwide, incidence and prevalence of autoimmunity have increased by 19.1% and 12.5% per year, respectively, over the past 30 years.41 Epidemiological research has associated vitamin D levels with many autoimmune conditions.42 However, only a few autoimmune disorders have a significant body of intervention trials to establish whether vitamin D supplementation confers a benefit.
Chronic urticaria (CU)
Chronic urticaria is characterised by continuous wheals, with or without angioedema (a swelling underneath the skin), lasting for more than 6 weeks. Whilst its exact causes are unknown, it is thought to be an autoimmune condition.43
Several studies, including both double-blind randomised, controlled and uncontrolled studies, have evaluated vitamin D supplementation in patients with CU and all of them found significant improvements in urticaria severity and/or quality of life, either against placebo or baseline (in uncontrolled studies).14,43,44,45,46 The severity of symptoms was reduced by 50% or more in some studies.44,45 Most studies included patients with VDD, but benefits have also been seen in patients without VDD.46
Two studies also found improvements in inflammatory markers,14,44 although this only reached statistical significance in one of them.14
Dosing regimens have varied widely in these studies, from 600 IU daily to 300 000 IU per month (equivalent to about 10 000 IU per day), and positive effects were observed with all regimens. Study durations ranged from 8 to 12 weeks. Where low versus high doses of vitamin D were compared, the higher dose achieved better results.43,46
Based on current research, a dose of 4000 IU per day orally, either taken daily or at greater intervals, could be suggested for people with CU, in particular for patients with low levels of vitamin D, whilst monitoring vitamin D status regularly.
Inflammatory bowel disease (IBD)
Inflammatory bowel disease is an auto-inflammatory condition characterised by inflammation of the gastrointestinal tract (GIT), and encompasses mainly Crohn’s disease (CD; which can affect any part of the GIT) and ulcerative colitis (UC; which only affects the colon). VDD is common in patients with IBD, although it is unclear whether this is a cause or a consequence of the disease.47
Two recent reviews and meta-analyses, one reviewing 12 and one 18 RCTs, evaluated the benefits of vitamin D supplementation on vitamin D levels and clinical outcomes.47,48 Although not all studies could be included into the meta-analyses, both reviews concluded that vitamin D supplementation led to significant improvements in disease severity and inflammatory markers,47 and in relapse rate,48 respectively, but in the latter study improvements in high-sensitivity C-reactive protein (hsCRP) failed to reach statistical significance and no improvements were seen in erythrocyte sedimentation rate (ESR; a non-specific measure of inflammation).
An RCT in both UC and CD patients found that low-dose vitamin D supplementation (500 IU per day) for 6 months significantly reduced the incidence of respiratory tract infections (RTIs) but not influenza in those with vitamin D levels below 20 ng/ml.49 Interestingly, this study found a worsening of UC symptoms in those patients with vitamin D levels of over 20 ng/ml at baseline, whilst no change in disease severity was observed in any other subgroup.
Several more recent studies found significant improvements in UC disease activity and severity, quality of life, oxidative stress, markers of angiogenesis (the formation of blood vessels, which is involved in the disease process) and some, but not all, inflammatory markers with vitamin D supplementation.50,51,52,53,54 Effective dosages have ranged from 2000 IU per day to a single dose of 300 000 IU intramuscularly and a daily dose of 60 000 IU for 8 days, with duration of follow-up being mostly 3 months.
A double-blind, placebo-controlled trial found no benefits of vitamin D, 25 000 IU per week for 26 weeks, on recurrence rate in patients with CD who had undergone a resection of part of their GIT.55 Mean vitamin D levels at baseline were in the insufficient range, 42 nmol/l and 43 nmol/l in the vitamin D and placebo groups, respectively, and almost doubled in the vitamin D group upon supplementation.
Overall, the evidence suggests a benefit of vitamin D supplementation in IBD, especially UC, with a range of dosage regimens showing good results. One should consider at least 2000 IU per day whilst monitoring vitamin D levels to ensure patients achieve adequate levels.
IBD in children
In children, IBD can lead to malnutrition and impaired bone formation.56
Several RCTs in children with IBD have found benefits of vitamin D supplementation in terms of disease severity, inflammatory markers and bone mineral density (BMD).57,58,59,60 A number of studies have evaluated the efficacy of ‘stoss’ therapy (single high dose) in this population, and have found this regimen to be safe and effective in raising vitamin D levels, and improved clinical outcomes were reported.58,61,62 One study compared 50 000 IU per week for 6 weeks with a single dose 300 000 IU vitamin D and found significantly higher levels of vitamin D at 12 weeks with the weekly regimen, 40.4 ng/ml versus 29.8 ng/ml.61 Another study found vitamin D levels exceeded the safe level of 250 nmol/l in four out of 23 children who received stoss therapy (dose dependent on age) at 1 month, but no symptoms of vitamin D toxicity were observed in any of the children, including one of 20 children whose calcium level was determined and who developed elevated calcium levels at 2 weeks, which normalised again 10 days later.58 Two studies found significant improvements with 2000 IU per day in children with or without VDD,57,59,60 despite the fact that in one of them less than 10% of children reached vitamin D levels of > 32 ng/ml.60
Overall, vitamin D supplementation at a dose of at least 2000 IU per day in children with IBD appears to be warranted, especially in children who are VDD. Weekly dosing or greater dosing intervals also appear to be beneficial and safe.
Multiple sclerosis (MS)
Multiple sclerosis is an autoimmune disease affecting the central nervous system through a reaction against the myelin sheaths that protect nerve cells and are important for signal transmission along neurons. Symptoms include fatigue, visual disturbances (through affecting the optic nerve), paraesthesia (abnormal sensation of the skin), muscle spasms, weakness and stiffness, pain and mobility problems, and can lead to severe disability. Epidemiological studies have shown an inverse relationship between vitamin D status and disability in patients with MS, i.e. the higher the vitamin D status the lower the disability.63
Three recent meta-analyses of six to 12 RCTs have evaluated the effectiveness of vitamin D supplementation in patients with MS, and have found no benefits on disability score, relapse rates or radiological signs.64,65,66 In fact, one meta-analysis found a worsening of relapse rate in the vitamin D compared with the control group.65 Dosages in most trials were 4000 IU per day or higher, up to 40 000 IU per day, for durations of 6 months or more.
A recent double-blind study not included in the above meta-analyses also found no improvements in clinical or radiographical measures with either high- or low-dose supplementation (20 400 IU every other day versus 400 IU every other day), despite the fact that vitamin D levels were raised to three times the level in the high-dose group (65 ng/ml versus 22 ng/ml).67
The RCTs looking at biomarkers of inflammation or MS-specific markers on the whole have also not found any consistent benefits of vitamin D supplementation.68,69,70,71
At this point the evidence suggests that vitamin D supplementation, especially of high doses, is of no benefit in MS, a surprising finding in view of the epidemiological data and popularity of vitamin D supplementation within parts of the MS community.
Psoriasis
Psoriasis is an autoimmune condition of the skin characterised by excessive proliferation of cells in the epidermis, which leads to red, flaky patches of skin covered with silvery scales. Topical vitamin D or vitamin D analogues have been used in the treatment of psoriasis since the 1990s, and this is backed up by a significant body of clinical research.72 For oral vitamin D, on the other hand, there are only a handful of clinical trials.
A meta-analysis of four double-blind, placebo-controlled trials found no benefit of oral vitamin D on psoriasis severity.73 Only one of the individual studies saw significant benefits over placebo. This trial used 60 000 IU every other week for 6 months,74 whilst two of the other studies used a monthly regimen of 100 000 IU and one a very low dose (40 IU per day).
Overall, the current evidence does not support the use of oral vitamin D for the management of psoriasis, although different dosing regimens, for example daily supplementation, have not been explored in clinical trials.
Rheumatoid arthritis (RA)
Rheumatoid arthritis is characterised by an autoimmune attack against the synovial cells, the cells lining the joints, causing painful, swollen and stiff joints, and can over time lead to joint damage. Epidemiological research has shown an inverse relationship between vitamin D levels and disease activity, and that vitamin D levels are lower in patients with RA than in healthy controls.75 High-dose vitamin D, up to 600 000 IU per day, has been used to treat RA as early as the 1930s, although toxicities have often been observed.76
A 2020 meta-analysis of six trials involving 438 patients with RA found significant improvements in Disease Activity Score 28 (DAS28), tender joint count and ESR, whilst improvements in pain visual analogue scale (VAS), swollen joint count and CRP (an inflammatory marker) failed to reach statistical significance.77 Another meta-analysis of five RCTs found a reduction of recurrence (borderline significance), whilst improvements in DAS failed to reach statistical significance and no improvement was seen in VAS.78 Most studies used weekly or monthly bolus dosages of 50 000−100 000 IU for durations of 12 or 24 weeks.
Two open-label RCTs have been published since, one showing no difference in disease activity or BMD in patients with idiopathic juvenile arthritis receiving 2000 IU per day for 24 weeks,79 whilst the other found a significant improvement in pain relief with 60 000 IU per week alongside calcium, 1000 mg per day, over calcium on its own in treatment-naïve patients with RA.80
Although research results are inconsistent, overall vitamin D supplementation appears to be beneficial for the management of RA.
Systemic lupus erythematosus (SLE)
Systemic lupus erythematosus is a systemic autoimmune condition that is characterised by inflammation in several tissues and organs, especially joints, skin, kidneys and blood vessels, potentially causing significant damage in these organs. Epidemiological and animal research suggests that vitamin D plays an important role in SLE,81,82 and the use of vitamin D for the treatment of lupus was first reported in the 1940s.83
A 2019 review and meta-analysis of five RCTs found no effects of vitamin D supplementation on disease activity (four trials) or anti-dsDNA (a marker of SLE, three trials), whilst two trials showed a decrease in fatigue.82
Two RCTs not included in the review found benefits of vitamin D either alone84 or with calcium85 on bone health, which is commonly negatively affected in patients with SLE. The former study also looked at disease activity and immune markers, but found no statistically significant changes. In both studies, all or most patients were vitamin D insufficient at baseline. Interestingly, two studies looked at the effect of different dosages on vitamin D levels in these patient populations and found that even with 50 000 IU per week only 75% of patients reached sufficiency levels, and less with lower dosages, suggesting that patients with SLE may require higher dosages, or different regimens, for full benefit.
Although evidence is limited, it would be advisable to recommend vitamin D supplementation to patients with SLE who are deficient, to ensure adequate levels for bone health. Vitamin D levels should be monitored to ensure supplements sufficiently raise vitamin D levels.
Autoimmune thyroid disease
There are two main forms of autoimmune thyroiditis (AIT): Hashimoto’s thyroiditis (HT) and Grave’s disease (GD). They are characterised by elevated levels of thyroid peroxidase antibodies (TPO-Ab) and/or thyroglobulin antibodies (Tg-Ab), which lead to dysfunction or destruction of the thyroid gland. Epidemiological research has shown that people with AIT have lower levels of vitamin D than healthy controls.86
A 2018 meta-analysis of six RCTs on the use of vitamin D in AIT, including HT and GD, found a significant lowering of thyroid antibodies, TPO-Ab and Tg-Ab, after 6 but not after 3 months.86 Dosages used in the included studies ranged from 1000 IU daily to 60 000 IU weekly, and durations were 1−6 months. Two of the studies used calcitriol, the activated form of vitamin D at 0.25 µg per day.
One more recent open-label RCT of 23 patients with HT found that vitamin D at 60 000 IU weekly for 8 weeks followed by the same dose monthly for another 4 months actually increased TPO-Ab, although it improved markers of thyroid function, with a decrease in thyroid-stimulating hormone (TSH) and an increase in free thyroxine (fT4).87 The authors of this study state that the cause for the increase of TPO-Ab in this study is unknown.
Overall, the evidence suggests that vitamin D supplementation is of benefit in patients with autoimmune thyroid disease, at least in those patients with insufficient vitamin D levels, with a dose of at least 2000 IU per day for 6 months having shown benefits.86
Bone health
As discussed in the section ‘Introduction’, vitamin D was first recognised for its importance in bone health, with the classic VDD diseases being rickets in children and osteomalacia in adults. A lot of research has therefore focussed on the role of vitamin D in bone health, in particular in the prevention and treatment of osteoporosis and fractures.
Osteoporosis, BMD and risk of fractures
Osteoporosis is characterised by low bone mass and structural deterioration of bone tissue, leading to bone fragility and an increased risk for fractures, commonly of the wrists, spine or hip, although any bone can be affected. The World Health Organization (WHO) defines osteoporosis as a BMD of 2.5 standard deviations below the mean peak mass (average of young healthy adults) as measured by a dual-energy X-ray absorptiometry (DEXA) scan. DEXA scans, however, only measure BMD and do not assess the structural micro-architecture of the bone tissue, and therefore may not accurately reflect risk of fractures.88 There are well over 50 RCTs looking into the role of vitamin D either alone or with calcium in osteoporosis.
In 2014, a Cochrane review of 53 RCTs involving 91 791 older adults concluded that there is high-quality evidence for the benefits of vitamin D and calcium, but not vitamin D on its own, for the prevention of non-vertebral or any type of fracture.89
Several meta-analyses into the benefits of vitamin D with or without calcium have been published since with conflicting results. Whilst some find a benefit of supplementation with vitamin D plus calcium,90,91,92 others did not find any benefits of either vitamin D, calcium or a combination of the two.93,94,95
In most studies investigating a combination of vitamin D and calcium, dosages ranged from 400 to 1000 IU and 500 to 1200 mg per day, respectively, whilst trials investigating vitamin D on its own commonly used intermittent high doses.90
Some of the meta-analyses carried out subgroup analyses looking at high and low dosages of both vitamin D and calcium, but found no effects,93,95 whilst others concluded that the minimum effective doses are 1200 mg calcium and 800 IU vitamin D per day.91
A couple of meta-analyses specifically looked at BMD and found improvements with combinations of vitamin D and calcium,94,96 although this was judged to be clinically irrelevant in one of the papers.94 As with studies on fractures, dosages were mostly in the ranges of 500−1200 mg calcium and 400−1000 IU vitamin D per day.
Overall, although the evidence from clinical trials is contradictory, in view of the excellent safety profile of vitamin D and calcium, supplementing a combination of the two, at dosages of 1000−1200 mg calcium and 400−1000 IU vitamin D per day, appears to be prudent for people at risk of fractures.
Cancer
A recent review of six meta-analyses concluded that “observational evidence indicates that low vitamin D status is associated with a higher risk of cancer outcomes, randomised trials show that vitamin D supplementation reduces total cancer mortality, but not cancer incidence”.97
Breast cancer
Epidemiological studies have shown that women with breast cancer are more likely to have low vitamin D levels at time of diagnosis compared with healthy controls.98
A 2020 meta-analysis of eight RCTs, involving 72 275 women, found no effect of vitamin D, either alone or with calcium, on the incidence of breast cancer.99 None of the included RCTs individually found a statistically significant reduction either, with dosages varying widely from 400 IU per day to 200 000 IU monthly and follow-up periods of 1−12 years.
A number of studies have also looked at a variety of biomarkers in women with breast cancer. Whilst improvements have been seen in some markers, including biomarkers for angiogenesis,100 total antioxidant capacity101 and 27-hydroxycholesterol (27HC; which is involved in the development of oestrogen-positive breast cancer),102 no significant changes were seen in others, including tumour proliferation or apoptosis biomarkers103 and inflammatory markers.101
At present the evidence does not support the use of vitamin D for prevention of breast cancer, which is surprising in view of the epidemiological data.98 And whilst there is evidence that vitamin D exerts positive effects on some breast cancer biomarkers, there is insufficient research to make recommendations for patients with breast cancer.
Colorectal cancer (CRC)
Colorectal cancer is the third most common cancer worldwide, and is significantly more common in developed countries.104 The role of vitamin D in the development of CRC has been controversial because of conflicting findings from supplementation and epidemiological studies.105
A review and meta-analysis including 166 studies and 854 195 participants evaluated the associations between vitamin D and CRC.104 Both vitamin D level and vitamin D intake (from diet and supplements) were associated with a significantly decreased risk of getting CRC, and higher vitamin D levels significantly decreased CRC and overall mortality. The data suggested that each 200 IU per day increase in total vitamin D intake was associated with a 10% decrease in the risk of colorectal adenoma (a pre-cancerous condition) and a 5% decrease in the risk of CRC. Similarly, a meta-analysis just focussing on vitamin D intervention trials found that vitamin D significantly improved progression-free survival and reduced adverse CRC outcomes by 30%,105 with the best results seen in a study using 4000 IU per day for 23 months.106 In the same publication, a reduction in CRC incidence failed to reach statistical significance. Another phase 3 clinical trial is currently underway to confirm these results.107
The evidence suggests that supplementation with vitamin D is beneficial both in the prevention of CRC and in improving outcomes in patients with CRC. Whilst dosages and regimens have varied between studies, 4000 IU per day has been shown to be beneficial.106
Prostate cancer
Prostate cancer is the second most common cancer in men. Vitamin D has been found to be involved in regulating hormone function in clinical and in vitro studies.108
A 2019 meta-analysis of six RCTs showed no effects of vitamin D on prostate-specific antigen (PSA; a marker of prostate cancer) or prostate cancer mortality.108 However, five of these studies did not use vitamin D3, but instead used vitamin D analogues or vitamin D2. The study that used vitamin D3 found a significant PSA response with 10 000 IU per day for 3−8 weeks.109 The 2019 meta-analysis also reviewed 16 uncontrolled trials that showed a modest benefit of vitamin D on PSA response rate, with 19% of patients having a reduction of at least 50%.108 Most of these studies used the activated calcitriol form of vitamin D.
Whilst research on vitamin D3 itself in prostate cancer is limited, there is some evidence that a high dose may be beneficial.
Skin cancer
Sun exposure is an important risk factor for skin cancers. Seeing that sun exposure is also our main natural source of vitamin D, it is not surprising that epidemiological studies have shown an increased risk of melanoma, keratinocyte and basal cell carcinoma, although not with squamous cell carcinoma, with higher vitamin D levels.110 Vitamin D intake through diet and/or supplements, on the other hand, is not associated with increased risks of skin cancers, except for basal cell carcinoma.110
Evidence for any effect of supplemental vitamin D from RCTs is currently lacking.
Chronic obstructive pulmonary disease (COPD)
Chronic obstructive pulmonary disease is a group of conditions, including emphysema and chronic bronchitis, which cause difficulties breathing. They tend to gradually get worse, can impair daily living and are amongst the top 10 causes of death globally.111
In 2020, a comprehensive review and meta-analysis of 25 RCTs involving 2670 patients found that supplementation with vitamin D or vitamin D analogues led to significant improvements in COPD assessment test score, lung function, sputum and 6-minute walk distance, and a halving in numbers of exacerbations.111 Looking at the results of the individual trials suggests that vitamin D analogues and vitamin D alongside vitamin A were more effective than vitamin D on its own.
Two much smaller meta-analyses found conflicting results. One involving eight studies and 687 patients found no significant improvements in lung function, but showed significant heterogeneity.112 The other one reviewed three trials with 469 patients and found a halving of moderate to severe exacerbations in patients with COPD with prior VDD, although no statistically significant overall improvement.113
Whilst the evidence is conflicting for vitamin D in patients with COPD, it seems prudent to ensure patients are not VDD, but there are insufficient data to suggest specific dosing regimens.
Cardiovascular disease (CVD) and risk factors
Cardiovascular disease is a general term for diseases affecting the heart and blood vessels, and is one of the main causes of death globally.114 CVD usually develops over many years, and risk factors include high blood pressure, abnormal blood lipids, smoking and poor diet.114
A meta-regression analysis of 22 RCTs involving 83 200 participants found no statistically significant reduction of non-fatal myocardial infarction, cardiac death, coronary heart disease events or stroke in vitamin D supplementation trials, with no heterogeneity and none of the individual trials showed significant effects.115
By far the biggest intervention trial, the VITAL trial in the USA, randomised 25 871 participants, men 50 years of age or older and women 55 years of age or older, to either vitamin D, 2000 IU per day, or placebo with a median follow-up of 5.3 years, and found no reduction in cardiovascular events.116
A very comprehensive meta-analysis of 81 studies overall looked at different cardiovascular risk factors, and found significant benefits of vitamin D supplementation for systolic and diastolic blood pressure, hsCRP, total, low-density lipoprotein (LDL) and high-density lipoprotein (HDL) cholesterol, and triglycerides.117 Subgroup analysis showed better outcomes when vitamin D levels of ≥ 86 nmol/l were achieved, with doses ≥ 4000 IU per day, trial durations of less than 6 months, and in obese people, although the impact of these factors had varied effects on different risk factors.
Blood pressure
A number of meta-analyses have looked at the effects of vitamin D on blood pressure with contradictory findings. Whilst one review found no benefits,118 others saw improvements in specific subgroups: in patients with hypertension and VDD at baseline,119,120,121 or together with calcium in younger adults.122 Most studies showed significant heterogeneity, and only one found dosage to be a significant factor, with dosages of 5000 IU per day or more being more effective.
Overall, the evidence for effects of vitamin D on blood pressure is mixed, but supplementation may decrease blood pressure in patients with hypertension and VDD, although the available data do not suggest an effective dosing regimen.
Cardiometabolic risk factors
A 2019 meta-analysis of 41 RCTs involving 3434 subjects looked at blood lipids and found significant improvements in total and LDL cholesterol and triglycerides, which were more pronounced in those with VDD at baseline.123
Another 2019 meta-analysis of eight trials and 305 subjects with CVD found significant improvements in HDL cholesterol, fasting glucose, insulin and Homeostatic Model Assessment of Insulin Resistance [HOMA-IR; a measure of insulin resistance (IR)], CRP, but not LDL, total cholesterol or triglycerides.124
Although results are mixed, vitamin D supplementation may help reduce cardiometabolic risk factors, especially in people with VDD.
Endothelial function
Three recent meta-analyses looked at the effect of vitamin D on endothelial function and found contradictory results. One meta-analysis of 26 RCTs involving 2808 subjects found no improvements in flow-mediated dilation, pulse wave velocity or central augmentation index (three markers of endothelial or arterial function).125 The authors carried out a subgroup analysis, but did not find any effects of dosing regimen or vitamin D status at baseline (overall, 42% of subjects were VDD or insufficient). Looking at the individual studies though, it appears that those that used monthly bolus doses of 100 000 IU or more found significant improvements.
The two other meta-analyses,126,127 on the other hand, found significant improvements, although in one improvements were limited to diabetics.127 Again, dosing was reported as not affecting the outcomes.127
Overall, evidence for the use of vitamin D for reduction of cardiovascular risk factors is contradictory, but maintaining adequate serum levels of vitamin D may confer some benefit, although this does not seem to translate into
a reduction in risk for cardiovascular events.
Depression
Three recent meta-analyses looked at the benefits of vitamin D in patients with depression. One of them reviewed 25 trials with a total of 7534 participants and found a positive effect on negative emotions, with patients with a diagnosis of major depressive disorder and VDD benefiting most.128 Another meta-analysis also reported benefits for depression (based on nine RCTs) and sleep (two RCTs), with all individual studies included showing some benefit.129 In the third meta-analysis (10 RCTs involving 1393 participants),130 improvements failed to reach statistical significance but there was significant heterogeneity within the results, which did not appear to be related to dosing regimen.
Since then more clinical trials have been published and have confirmed the benefits of vitamin D supplementation on depressive symptoms and/or anxiety.131,132,133
Overall, the evidence suggests that vitamin D supplementation is of benefit for patients with depression, in particular those with VDD. Dosing regimens that have shown benefits varied from 1000 IU per day to 100 000 IU weekly for 8 weeks, and a single bolus dose of 300 000 IU with 12-week follow-up.
Type 2 diabetes mellitus (T2DM)/ glycaemic control
Epidemiological studies suggest a role of vitamin D in glycaemic control of patients with T2DM.134
Although there is some heterogeneity amongst studies, over the past 5 years a number of meta-analyses, covering over 20 RCTs overall, have reported benefits of vitamin D supplementation on glycosylated haemoglobin (HbA1c; a long-term measure of blood glucose control), fasting blood glucose (FBG) and/or HOMA-IR, especially in patients with VDD.135,136,137,138 Only one meta-analysis found no statistically significant improvements in HbA1c (no other variables were assessed).134
When it comes to glycaemic control in non-diabetics at risk of IR, results are more contradictory, with one meta-analysis (12 RCTs) finding significant reductions in FBG, HOMA-IR and circulating levels of insulin,139 whilst another found no benefits on IR,140 and yet another benefits for FBG and HbA1c but not HOMA-IR.141 One meta-analysis found no overall effect on FBG, IR or preventing T2DM, but noted that results differed significantly between different subgroups.142 One meta-analysis found that vitamin D reduced the risk of developing T2DM in non-obese but not obese people with prediabetes.143 Overall, these studies suggested that dosages of 2000 IU or more per day were more beneficial than lower dosages.
Overall, the evidence suggests that vitamin D may improve glycaemic control in people with both T2DM and prediabetes, especially those with VDD. Benefits have been seen with a range of dosing regimens, and there is some evidence that dosages of 2000 IU per day or more give better benefits.
It is thought that the ability of vitamin D to reduce inflammation and oxidative stress may mediate, at least in part, its benefits in T2DM and prediabetes.144 Intervention studies have confirmed that vitamin D supplementation decreases inflammatory and oxidative stress markers in patients with T2DM.144,145,146
Muscle strength
Vitamin D deficiency has been associated with muscle aches and weakness.147
Vitamin D supplementation has therefore been trialled for increasing strength in various populations. Whilst benefits of vitamin D have been observed in healthy adults,148 no benefits have been seen in athletes147,149 or postmenopausal women.150,151 Findings in elderly people are mixed, with some studies showing small improvements in some strength tests,152 whilst others show no additional benefit of vitamin D in addition to that of exercise.153
Non-alcoholic fatty liver disease (NAFLD)
Non-alcoholic fatty liver disease is caused by a build-up of fat within liver cells, and is closely associated with abnormal glucose and lipid metabolism, and increased inflammation and oxidative stress.154 People with NAFLD tend to have lower levels of vitamin D.155
Four recent meta-analyses have evaluated the potential benefits of vitamin D in patients with NAFLD, reviewing six to 10 studies each, with some overlap of included studies. Two found no improvements in liver enzymes (markers of liver damage), lipid or glucose metabolism.154,155 One review found significant improvements in alkaline phosphatase, but not in other liver enzymes.156 The fourth one found significant improvements in glucose metabolism, alanine aminotransferase (a liver enzyme) and triglycerides, but not cholesterol (total, LDL or HDL) or aspartate aminotransferase (another liver enzyme).157 One of the studies carried out a subgroup analysis, but found no effects of dosing regimen, vitamin D status at baseline or other factors.154 Looking at the individual study characteristics and results also does not show any obvious associations.
Two more RCTs have been published since, and both found significant improvements in blood markers158,159 and transient elastography (a specific ultrasound scan).158 Dosages used in these trials were 1000 IU per day for 12 months and 50 000 IU per week for 3 months.
Whilst findings from clinical trials are inconsistent, there may be a benefit of vitamin D in patients with NAFLD, but the research to date does not allow to make any specific dose recommendations.
Obesity
Obesity has become a major health concern, and in 2017−2018 the prevalence of obesity in adults in the USA was 42.4%, with 9.2% being severely obese.160 Epidemiological research has shown that people with obesity have lower levels of vitamin D, and that there is a dose−response relationship between the two.161 However, it is unclear which is cause and which is effect.
A number of clinical trials have looked at the effect of vitamin D on weight and body composition. A meta-analysis in 2019 found that vitamin D, when included in weight loss programmes, had small, but statistically significant additive effects on body mass index (BMI; average decrease −0.3) and waist circumference (average decrease −1.4cm), but not weight.162 Two other meta-analyses found no effect of vitamin D supplementation on body fat163 or BMI, weight or fat mass.164
Adipokines, including leptin and adiponectin, are cell-signalling proteins secreted by fat cells, and they play an important role in obesity. A recent meta-analysis reviewed studies looking at vitamin D supplementation and adipokines, but found no effects.165
Overall, the evidence suggests that vitamin D does not help with weight or fat loss as such. However, because people with obesity are at increased risk for many conditions associated with VDD, supplementation should be considered, especially in those with VDD. Vitamin D levels should be monitored and vitamin D dose adjusted accordingly, as studies have shown that obesity decreases the effect of vitamin D supplementation.11
Pain
Epidemiological studies have shown that patients with arthritis, muscle pain and chronic widespread pain have lower vitamin D levels than people without these painful conditions.166
Two meta-analyses looking at low back pain167 and non-specific musculoskeletal pain168 found no benefit of vitamin D supplementation, although the authors of one of the reviews noted that this is based on poor-quality evidence.167 On the other hand, a meta-analysis of RCTs on chronic widespread pain, such as in fibromyalgia syndrome, found a halving of pain scores with vitamin D supplementation.169 Benefits were also reported in a meta-analysis of different types of chronic pain, although benefits were only seen in clinical trials carried out in a hospital (as opposed to a community) setting.170 The authors of the latter review hypothesize that a possible explanation for that discrepancy may be that higher dosages were used in hospital-based trials.
Overall, the evidence regarding the potential benefits of vitamin D for pain-related conditions is mixed and may depend on the type of pain.
Polycystic ovary syndrome (PCOS)
Polycystic ovary syndrome is a common condition that affects about one in 10 women, and is characterised by enlarged ovaries that contain many fluid-filled sacs (follicles) that surround the eggs, excess androgens (‘male’ hormones) and irregular periods.171 Whilst generally considered a gynaecological condition, the underlying cause of PCOS is thought to be IR. PCOS has been associated with low vitamin D levels.172
There is a significant body of research into various markers of PCOS, which have been summarised in several recent meta-analyses. Vitamin D supplementation has been shown to improve blood sugar and lipid metabolism,173,174,175 androgen levels,172,174 inflammatory and oxidative stress markers,176 and follicular development and menstrual cycle regulation.177
Fertility problems are common in women with PCOS. Three recent clinical trials also showed improvements in a number of fertility-related markers178,179,180 and pregnancy rates.180
Overall, the evidence shows benefits for women with PCOS with regards to metabolic parameters, hormone balance and fertility. The three fertility-related studies used approximately 3000 IU per day, either as a daily or weekly supplement, dosages in other studies varied widely with both daily and bolus regimens.
Pregnancy
Pregnant women and new-borns are at increased risk of VDD,181 raising the question whether vitamin D supplementation during pregnancy offers clinical benefits.
Gestational diabetes mellitus (GDM)
Gestational diabetes mellitus carries significant risks to both mother and baby, and is increasing worldwide.182
Five meta-analyses, including over 20 RCTs, have evaluated the potential benefits of vitamin D for pregnant women with GDM, and all have found significant improvements in glycaemic control and/or reduced adverse maternal or neonatal outcomes.182,183,184,185,186,187 A wide range of dosing regimens have been used, from 400 IU daily to single bolus doses of 300 000 IU. Looking at the individual study results of the most comprehensive meta-analysis that included 19 RCTs,183 positive results, although not always statistically significant, appeared to be consistent over a wide range of dosage regimens, with the exception of two studies that used 300 000 IU bolus dosages and showed no effects.
The evidence shows a clear benefit of vitamin D supplementation for both mother and baby in women with gestational diabetes. A wide range of dosages appeared to be beneficial, except large bolus doses.
Pre-eclampsia
Pre-eclampsia is a pregnancy-related condition, characterised by hypertension, proteinuria (protein in urine) and oedema, which is potentially life-threatening to both mother and baby.
Three recent meta-analyses, covering over 25 RCTs, all concluded that vitamin D supplementation reduced the risk of pre-eclampsia, with estimated risk reductions of 37−63%.188,189,190
Daily dosages have ranged from 200 IU to 5000 IU, but intermittent dosages have also been used, commonly 50 000 IU every 2 weeks. One meta-analysis suggests a dose−response relationship, with higher dosages being more protective.189
Overall, the evidence is in favour of vitamin D supplementation for reducing the risk of pre-eclampsia, with higher dosage being more effective.
Pregnancy outcomes
Three recent meta-analyses, covering over 20 RCTs, have shown vitamin D supplementation being of benefit in terms of birth weight.181,191,192 One of these studies also looked at mortality, and found significantly reduced mortality with vitamin D dosages of 2000 IU per day or lower, whilst reduction of mortality lost statistical significance with higher dosages.192 This study also showed that there was no association of vitamin D supplementation with congenital abnormalities.
Overall, supplementing 2000 IU per day of vitamin D during pregnancy appears to be beneficial for both maternal and neonatal outcomes.
Respiratory tract infections
Respiratory tract infections are a major cause of morbidity and mortality globally. Low levels of vitamin D have been associated with an increased susceptibility to RTIs.193
Two recent meta-analyses, covering more than 40 RCTs, have shown that daily and weekly, but not bolus, administration of vitamin D reduces the risk of RTIs.193,194 Whilst one study found that these effects were stronger in patients with baseline vitamin D levels of below 25 ng/ml,193 the other found better effects in children aged 1−15 years, and dosages between 400 and 1000 IU per day.194
COVID-19
In view of the benefits of vitamin D in reducing the risk of other RTIs, Vitamin D has received much attention as a possible factor in the incidence and severity of COVID-19. Epidemiological studies looking at vitamin D levels and the risk of getting COVID-19 have mostly shown that VDD is associated with a higher risk of getting COVID-19, with an up to 80% increased risk reported,195,196,197,198 although Pereira et al. found no association.199 Three meta-analyses looked into the risk of severe COVID-19, and found an increased risk of up to 260% in those who were VDD.197,198,199
A number of RCTs have also been conducted. One RCT in asymptomatic or mildly symptomatic patients found that those who received vitamin D, 60 000 IU per day for 7 days, were more likely to have recovered within 21 days [defined as a negative polymerase chain reaction (PCR) test] and had lower biomarkers associated with severe disease than those on placebo.200 One RCT in hospitalised patients found that a single dose of 200 000 IU did not reduce mortality, risk of admission to the intensive care unit (ICU), mechanical ventilation or length of hospital stay compared with placebo.201
On the other hand, a pilot RCT from Spain compared 76 patients hospitalised with COVID-19, some of whom received oral calcifediol (0.532 mg) on the day of admission, 0.266 mg on days 3 and 7, and then weekly until discharge or ICU admission in addition to standard care, whilst the controls received standard care only. Only 2% of patients on calcifediol needed transfer to the ICU and none of them died, compared with two deaths (8%) and 50% of patients needing ICU treatment in the control group.202 This raises the question, whether the use of an activated form of vitamin D may provide more benefit in acutely and severely ill patients in whom activation of vitamin D3 may be impaired or take too long.
A number of case series, prospective unrandomised and/or uncontrolled studies have also shown benefits of vitamin D supplementation in COVID-19 patients.203,204,205,206
Overall, whilst evidence is still emerging, ensuring adequate vitamin D levels appears to be a prudent approach for prevention of severe COVID-19 as well as RTIs in general, whilst administration of high-dose calcifediol should be considered in patients hospitalised with severe COVID-19. Griffin et al. recommend 4000 IU vitamin D per day for 1 month, followed by 800−1000 IU per day for maintenance in people at risk of VDD for prevention of COVID-19.207 This level of supplementation should also help prevent other RTIs.
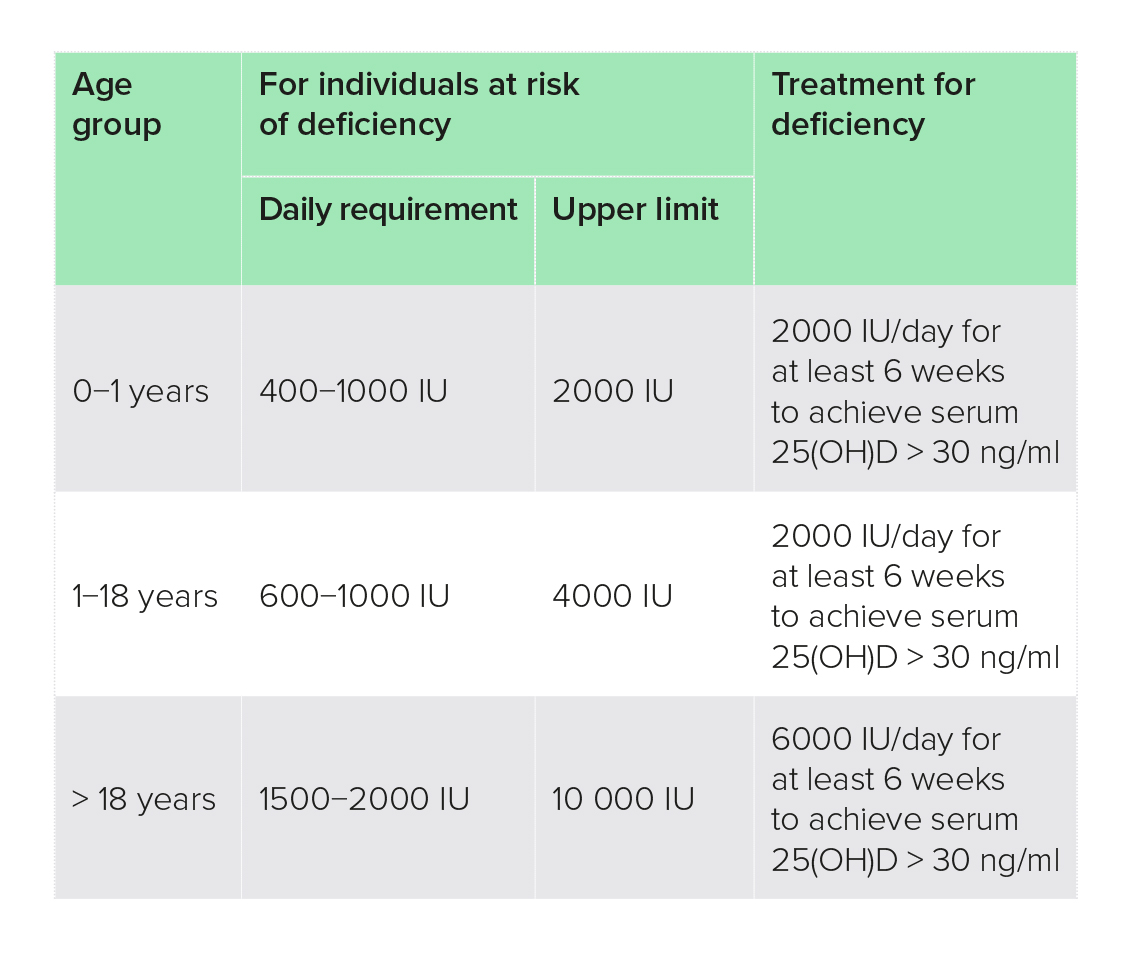
Table 2: Guidelines for vitamin D intake in VDD210
Safety
Based on its role in calcium metabolism, the main safety concern with excessive vitamin D levels is hypercalcaemia. Hypercalcaemia can lead to nausea, vomiting, muscle weakness, neuropsychiatric disturbances, pain, loss of appetite, dehydration, polyuria, excessive thirst, kidney stones and, in extreme cases, renal failure, calcification of soft tissues, cardiac arrhythmias and even death.6
Vitamin D has been used at a wide range of dosages, and up to 10 000 IU per day is considered to be safe,1,6 but the NIH sets the upper tolerable intake for children aged 9 years and older and adults as 4000 IU per day.6
A meta-analysis of 62 clinical trials with 19 389 participants reported that there was no increased risk of any or non-hypercalcaemic adverse events (AEs).208 The authors also reported that although gastrointestinal and skin AEs have been reported, these were not more common in the vitamin D as compared with the control groups. More participants withdrew from studies, which may indicate AEs, where calcium was taken by both the vitamin D and the control groups, but not in studies without calcium. The authors concluded that “vitamin D, by itself, does not increase the risk of non-calcemic adverse effects”. The authors carried out various subgroup analyses, and found no increase in AEs regardless of whether vitamin D was given with or without calcium, length of supplementation, baseline vitamin D levels or whether vitamin D2 or D3 were used, a subgroup analysis by dose of vitamin D was not reported.208
A Cochrane review of 53 RCTs on fracture risk involving 91 791 older adults found no increased mortality in patients receiving vitamin D with or without calcium.89 They found a small (4%) but statistically significant increased risk of gastrointestinal symptoms, especially when combined with calcium, and a 17% increased risk of kidney disease when taken with calcium, but a 41% reduced risk of kidney disease when taken without calcium. The overall risk increase for kidney disease was 16%, with an absolute risk increase from 1.69 to 1.98 in 1000. The risk of hypercalcaemia, which was usually mild (2.6−2.8 mmol/L), was more than doubled in people receiving vitamin D or an analogue compared with controls, with a more than four times higher risk in people receiving calcitriol.89
Interaction with medications6
Orlistat may decrease vitamin D absorption.
Statins suppress cholesterol synthesis and vitamin D is made from cholesterol, statins may therefore reduce vitamin D levels. Vitamin D may reduce the potency of statins.
Corticosteroids can impair vitamin D metabolism, and people on oral steroids have an increased risk of VDD.
Thiazide diuretics decrease urinary calcium excretion, which in combination with vitamin D supplements might lead to hypercalcaemia. Additional monitoring of vitamin D and calcium levels and possibly renal function should be instigated by the prescribing physician.
Cautions in specific conditions209
Due to its effect on calcium metabolism, vitamin D should be used with caution in patients with arteriosclerosis, histoplasmosis, hypercalcaemia, hyperparathyroidism, lymphoma, kidney disease, tuberculosis and sarcoidosis.
Pregnancy and lactation
Pregnant and lactating women are at particular risk of VDD, and therefore often advised to supplement vitamin D. The NIH sets the same upper tolerable limit (UTL) of 4000 IU per day for these groups.6
See also under the section ‘Pregnancy’ for studies reporting reduced mortality of infants and no risk of congenital abnormalities associated with vitamin D supplementation.
Children
Vitamin D supplementation in children is generally safe, and has shown benefits for a number of conditions, including IBD, allergies and prevention of respiratory infections (see above). The NIH set UTLs according to age groups:6
• Up to 6 months: 1000 IU per day
• 6−12 months: 1500 IU per day
• 1−3 years: 2500 IU per day
• 4−8 years: 3000 IU per day
• From 9 years: 4000 IU per day
Conclusion
Whilst epidemiological research has linked vitamin D with many conditions, it is important to remember that a statistical association does not necessarily reflect a causal relationship. Clinical supplementation trials have shown benefits of vitamin D in a number of conditions, including AD, CU, CRC, depression, PCOS, T2DM and pregnancy-related disorders. Some benefits have also been seen for cardiovascular risk factors, but studies looking at cardiovascular events found no reduction in risk. The reason for these contradictory findings is unknown. Interestingly, although epidemiological as well as preclinical research points strongly to an important role of vitamin D in autoimmunity, results from supplementation studies have either shown no benefits (MS, psoriasis) or have been contradictory (RA, autoimmune thyroid disease, SLE).
It is possible that vitamin D status is a proxy for other factors, in particular exposure to sunlight, which has also been shown to have other benefits. Contradictory results can also be due to differences in baseline vitamin D status and methodological problems with establishing vitamin D status, as well as to the large range of dosing regimens used in clinical trials. Further well-designed studies may find optimal dosing regimens as well as those populations who may benefit most.
From a clinical perspective, the best practice is to establish vitamin D status at the start of a programme and monitor regularly to achieve and maintain optimal levels, through appropriate sun exposure and/or supplementation.
Acknowledgements
Author contributions: K. Elgar carried out the literature review and formulated the manuscript.
Additional contributions: B. Brown contributed Tables 1 and 2.
Peer-reviewers and editors: the Nutritional Medicine Institute thanks the peer-reviewers and editors for their important contributions.
Funding: Open Access publication was supported by an unrestricted donation from Pure Encapsulations, Sudbury, MA, USA. No other funding or sponsorship has been received for this work.
Declaration of interest: K. Elgar has received consultancy fees from Pure Encapsulations, Sudbury, MA, USA. This article is the independent work of the author and Pure Encapsulations was not involved in the decision to publish this research.
References
1 Holick, M. F. (2007) Vitamin D deficiency. N. Engl. J. Med., 357, 266–281.
2 Bikle, D. D. (2014) Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol., 21, 319–329.
3 Hawk, J. L. M. (2020) Safe, mild ultraviolet-B exposure: An essential human requirement for vitamin D and other vital bodily parameter adequacy: A review. Photodermatol. Photoimmunol. Photomed., 36, 417–423.
4 Altieri, B. et al. (2020) Vitamin D testing: advantages and limits of the current assays. Eur. J. Clin. Nutr., 74, 231–247.
5 Charoenngam, N. & Holick, M. F. (2020) Immunologic effects of vitamin D on human health and disease. Nutrients, 12, 2097.
6 NIH (2021) Vitamin D Fact Sheet for Health Professionals. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/#h5.
7 NICE (2020) Vitamin D deficiency in adults − treatment and prevention. https://cks.nice.org.uk/topics/vitamin-d-deficiency-in-adults-treatment-prevention/.
8 Autier, P. et al. (2017) Effect of vitamin D supplementation on non-skeletal disorders: a systematic review of meta-analyses and randomised trials. Lancet. Diabetes Endocrinol., 5, 986–1004.
9 Alfredsson, L. et al. (2020) Insufficient sun exposure has become a real public health problem. Int. J. Environ. Res. Public Health, 17, 5014.
10 van der Rhee, H. J., de Vries, E. & Coebergh, J. W. (2016) Regular sun exposure benefits health. Med. Hypotheses, 97, 34–37.
11 de Oliveira, L. F., de Azevedo, L. G., da Mota Santana, J., de Sales, L. P. C. & Pereira-Santos, M. (2020) Obesity and overweight decreases the effect of vitamin D supplementation in adults: systematic review and meta-analysis of randomized controlled trials. Rev. Endocr. Metab. Disord., 21, 67–76.
12 Lieben, L. & Carmeliet, G. (2013) The delicate balance between vitamin D, calcium and bone homeostasis: lessons learned from intestinal- and osteocyte-specific VDR null mice. J. Steroid Biochem. Mol. Biol., 136, 102–106.
13 Fukumoto, S. (2014) Phosphate metabolism and
vitamin D. Bonekey Rep., 3, 497.
14 Mony, A. et al. (2020) Effect of vitamin D supplementation on clinical outcome and biochemical profile in South Indian population with vitamin D-deficient chronic urticarial − A randomized double-blind placebo controlled trial. Clin. Chim. Acta, 504, 1–6.
15 Rueter, K. et al. (2020) In ‘high-risk’ infants with sufficient vitamin D status at birth, infant vitamin D supplementation had no effect on allergy outcomes: a randomized controlled trial. Nutrients, 12, 1747.
16 Rosendahl, J. et al. (2019) High-dose vitamin D supplementation does not prevent allergic sensitization of infants. J. Pediatr., 209, 139−145.e1.
17 Yepes-Nuñez, J. J. et al. (2018) Vitamin D supplementation in primary allergy prevention: Systematic review of randomized and non-randomized studies. Allergy 73, 37–49.
18 Aryan, Z., Rezaei, N. & Camargo, C. A. J. (2017) Vitamin D status, aeroallergen sensitization, and allergic rhinitis: A systematic review and meta-analysis. Int. Rev. Immunol., 36, 41–53.
19 Kim, Y. H. et al. (2016) Vitamin D levels in allergic rhinitis: a systematic review and meta-analysis. Pediatr. Allergy Immunol., 27, 580–590.
20 Bakhshaee, M., Sharifian, M., Esmatinia, F., Rasoulian, B. & Mohebbi, M. (2019) Therapeutic effect of vitamin D supplementation on allergic rhinitis. Eur. Arch. Otorhinolaryngol., 276, 2797–2801.
21 Jerzyńska, J. et al. (2018) Clinical and immunological effects of vitamin D supplementation during the pollen season in children with allergic rhinitis. Arch. Med. Sci., 14, 122–131.
22 Shabana, M. A., Esawy, M. M., Ismail, N. A. & Said, A. M. (2019) Predictive role of IL-17A/IL-10 ratio in persistent asthmatic patients on vitamin D supplement. Immunobiology, 224, 721–727.
23 Riverin, B. D., Maguire, J. L. & Li, P. (2015) Vitamin D supplementation for childhood asthma: a systematic review and meta-analysis. PLoS One, 10, e0136841.
24 Martineau, A. R. et al. (2016) Vitamin D for the management of asthma. Cochrane Database Syst. Rev., 9, CD011511.
25 Jolliffe, D. A. et al. (2017) Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet Respir. Med., 5, 881–890.
26 Jaura, J., Kelsberg, G. & Safranek, S. (2020) Does vitamin D supplementation reduce asthma exacerbations? J. Fam. Pract., 69, E4–E6.
27 Thakur, C. et al. (2021) Vitamin-D supplementation as an adjunct to standard treatment of asthma in children: A randomized controlled trial (ViDASTA Trial). Pediatr. Pulmonol., doi:10.1002/ppul.25287.
28 Jat, K. R. et al. (2020) Efficacy of vitamin D supplementation in asthmatic children with vitamin D deficiency: A randomized controlled trial (ESDAC trial). Pediatr. Allergy Immunol., doi:10.1111/pai.13415.
29 Forno, E. et al. (2020) Effect of vitamin D3 supplementation on severe asthma exacerbations in children with asthma and low vitamin D levels: the VDKA randomized clinical trial. JAMA, 324, 752–760.
30 Alansari, K., Davidson, B. L., Yousef, K. I., Mohamed, A. N. H. & Alattar, I. (2017) Rapid vs maintenance vitamin D supplementation in deficient children with asthma to prevent exacerbations. Chest, 152, 527–536.
31 Ducharme, F. M. et al. (2019) Impact of two oral doses of 100,000 IU of vitamin D(3) in preschoolers with viral-induced asthma: a pilot randomised controlled trial. Trials, 20, 138.
32 Andújar-Espinosa, R. et al. (2020) Effect of vitamin D supplementation on asthma control in patients with vitamin D deficiency: the ACVID randomised clinical trial. Thorax, doi:10.1136/thoraxjnl-2019-213936.
33 Hattangdi-Haridas, S. R., Lanham-New, S. A., Wong, W. H. S., Ho, M. H. K. & Darling, A. L. (2019) Vitamin D deficiency and effects of vitamin D supplementation on disease severity in patients with atopic dermatitis: a systematic review and meta-analysis in adults and children. Nutrients, 11, 1854.
34 Kim, M. J., Kim, S.-N., Lee, Y. W., Choe, Y. B. & Ahn, K. J. (2016) Vitamin D status and efficacy of vitamin D supplementation in atopic dermatitis: a systematic review and meta-analysis. Nutrients, 8, 789.
35 Kim, G. & Bae, J.-H. (2016) Vitamin D and atopic dermatitis: A systematic review and meta-analysis. Nutrition, 32, 913–920.
36 Mansour, N. O. et al. (2020) The impact of vitamin D supplementation as an adjuvant therapy on clinical outcomes in patients with severe atopic dermatitis: A randomized controlled trial. Pharmacol. Res. Perspect., 8, e00679.
37 Imoto, R. R. et al. (2021) Vitamin D supplementation and severity of atopic dermatitis: pre-post assessment. Allergol. Immunopathol. (Madr.), 49, 66–71.
38 Lara-Corrales, I. et al. (2019) Vitamin D level and supplementation in pediatric atopic dermatitis: a randomized controlled trial. J. Cutan. Med. Surg., 23, 44–49.
39 Sánchez-Armendáriz, K. et al. (2018) Oral vitamin D3 5000 IU/day as an adjuvant in the treatment of atopic dermatitis: a randomized control trial. Int. J. Dermatol., 57, 1516–1520.
40 NIH Autoimmune Diseases Coordinating Committee (2005) Progress in autoimmune diseases research. https://www.niaid.nih.gov/sites/default/files/adccfinal.pdf.
41 Lerner, A., Jeremias, P. & Matthias, T. (2015) The world incidence and prevalence of autoimmune diseases is increasing. Int. J. Celiac Dis., 3, 151–155.
42 Murdaca, G. et al. (2019) Emerging role of vitamin D in autoimmune diseases: An update on evidence and therapeutic implications. Autoimmun. Rev., 18, 102 350.
43 Nabavizadeh, S. H., Alyasin, S., Esmaeilzadeh, H., Mosavat, F. & Ebrahimi, N. (2020) The effect of vitamin D add-on therapy on the improvement of quality of life and clinical symptoms of patients with chronic spontaneous urticaria. Asian Pacific J. Allergy Immunol., doi:10.12932/AP-021219-0705.
44 Ariaee, N., Zarei, S., Mohamadi, M. & Jabbari, F. (2017) Amelioration of patients with chronic spontaneous urticaria in treatment with vitamin D supplement. Clin. Mol. Allergy, 15, 22.
45 Oguz Topal, I. et al. (2016) Does replacement of vitamin D reduce the symptom scores and improve quality of life in patients with chronic urticaria? J. Dermatolog. Treat., 27, 163–166.
46 Rorie, A., Goldner, W. S., Lyden, E. & Poole, J. A. (2014) Beneficial role for supplemental vitamin D3 treatment in chronic urticaria: a randomized study. Ann. Allergy. Asthma Immunol., 112, 376–382.
47 Guzman-Prado, Y., Samson, O., Segal, J. P., Limdi, J. K. & Hayee, B. (2020) Vitamin D therapy in adults with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm. Bowel Dis., 26, 1819–1830.
48 Li, J., Chen, N., Wang, D., Zhang, J. & Gong, X. (2018) Efficacy of vitamin D in treatment of inflammatory bowel disease: A meta-analysis. Medicine (Baltimore), 97, e12662.
49 Arihiro, S. et al. (2019) Randomized trial of vitamin D supplementation to prevent seasonal influenza and upper respiratory infection in patients with inflammatory bowel disease. Inflamm. Bowel Dis., 25, 1088–1095.
50 Karimi, S. et al. (2020) Inflammatory biomarkers response to two dosages of vitamin D supplementation in patients with ulcerative colitis: A randomized, double-blind, placebo-controlled pilot study. Clin. Nutr. ESPEN, 36, 76–81.
51 Emami, M. R., Sharifi, A., Yaseri, M., Derakhshanian, H. & Hosseinzadeh-Attar, M. J. (2020) Vitamin D suppresses proangiogenic factors in patients with ulcerative colitis: A randomized double blind placebo controlled clinical trial. Complement. Ther. Clin. Pract., 39, 101 086.
52 Ahamed Z, R. et al. (2019) Oral nano vitamin D supplementation reduces disease activity in ulcerative colitis: a double-blind randomized parallel group placebo-controlled trial. J. Clin. Gastroenterol., 53, e409–e415.
53 Sharifi, A., Vahedi, H., Nedjat, S., Rafiei, H. & Hosseinzadeh-Attar, M. J. (2019) Effect of single-dose injection of vitamin D on immune cytokines in ulcerative colitis patients: a randomized placebo-controlled trial. APMIS, 127, 681–687.
54 Karimi, S. et al. (2019) The effects of two vitamin D regimens on ulcerative colitis activity index, quality of life and oxidant/anti-oxidant status. Nutr. J., 18, 16.
55 de Bruyn, J. R. et al. (2020) High-dose vitamin D does not prevent postoperative recurrence of Crohn’s disease in a randomized placebo-controlled trial. Clin. Gastroenterol. Hepatol., doi:10.1016/j.cgh.2020.05.037.
56 Benchimol, E. I. et al. (2007) Effect of calcium and vitamin D supplementation on bone mineral density in children with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr., 45, 538–545.
57 El Amrousy, D., El Ashry, H., Hodeib, H. & Hassan, S. (2020) Vitamin D in children with inflammatory bowel disease: a randomized controlled clinical trial. J. Clin. Gastroenterol., doi:10.1097/MCG.0000000000001443.
58 Martin, N. G., Rigterink, T., Adamji, M., Wall, C. L. & Day, A. S. (2019) Single high-dose oral vitamin D3 treatment in New Zealand children with inflammatory bowel disease. Transl. Pediatr., 8, 35–41.
59 Hradsky, O. et al. (2017) Supplementation with 2000 IU of cholecalciferol is associated with improvement of trabecular bone mineral density and muscle power in pediatric patients with IBD. Inflamm. Bowel Dis., 23, 514–523.
60 Pappa, H. M. et al. (2014) Maintenance of optimal vitamin D status in children and adolescents with inflammatory bowel disease: a randomized clinical trial comparing two regimens. J. Clin. Endocrinol. Metab., 99, 3408–3417.
61 Lee, R. et al. (2020) Single high-dose vitamin D3 supplementation in pediatric patients with inflammatory bowel disease and hypovitaminosis D. J. Pediatr. Gastroenterol. Nutr., 70, e77–e80.
62 Shepherd, D. et al. (2015) Single high-dose oral vitamin D3 therapy (stoss): a solution to vitamin D deficiency in children with inflammatory bowel disease? J. Pediatr. Gastroenterol. Nutr., 61, 411–414.
63 Moosazadeh, M. et al. (2021) Vitamin D status and disability among patients with multiple sclerosis: a systematic review and meta-analysis. AIMS Neurosci., 8, 239–253.
64 McLaughlin, L. et al. (2018) Vitamin D for the treatment of multiple sclerosis: a meta-analysis. J. Neurol., 265, 2893–2905.
65 Zheng, C., He, L., Liu, L., Zhu, J. & Jin, T. (2018) The efficacy of vitamin D in multiple sclerosis: A meta-analysis. Mult. Scler. Relat. Disord., 23, 56–61.
66 Doosti-Irani, A. et al. (2019) The effects of vitamin D supplementation on expanded disability status scale in people with multiple sclerosis: A critical, systematic review and metaanalysis of randomized controlled trials. Clin. Neurol. Neurosurg., 187, 105 564.
67 Dörr, J. et al. (2020) High-dose vitamin D supplementation in multiple sclerosis − results from the randomized EVIDIMS (efficacy of vitamin D supplementation in multiple sclerosis) trial. Mult. Scler. J. Exp. Transl. Clin., 6, 2055217320903474.
68 Azimi, A. et al. (2019) Effects of vitamin D supplements on IL-10 and INFγ levels in patients with multiple sclerosis: a systematic review and meta-analysis. Maedica, 14, 413–417.
69 Smolders, J. et al. (2020) Vitamin D(3) supplementation and neurofilament light chain in multiple sclerosis. Acta Neurol. Scand., 141, 77–80.
70 Holmøy, T. et al. (2019) Vitamin D supplementation and neurofilament light chain in multiple sclerosis. Acta Neurol. Scand., 139, 172–176.
71 Rolf, L. et al. (2018) Vitamin D(3) supplementation and the IL-2/IL-2R pathway in multiple sclerosis: Attenuation of progressive disturbances? J. Neuroimmunol., 314, 50–57.
72 Federman, D. G., Froehlich, C. W. & Kirsener, R. S. (1999) Topical psoriasis therapy. Am. Fam. Physician, 59, 957–962.
73 Theodoridis, X. et al. (2021) Effectiveness of oral vitamin D supplementation in lessening disease severity among patients with psoriasis: A systematic review and meta-analysis of randomized controlled trials. Nutrition, 82, 111 024.
74 Disphanurat, W., Viarasilpa, W., Chakkavittumrong, P. & Pongcharoen, P. (2019) The clinical effect of oral vitamin D2 supplementation on psoriasis: a double-blind, randomized, placebo-controlled study. Dermatol. Res. Pract., 2019, 5237642.
75 Lin, J., Liu, J., Davies, M. L. & Chen, W. (2016) Serum vitamin D level and rheumatoid arthritis disease activity: review and meta-analysis. PLoS One, 11, e0146351.
76 Lyons, B. H. & Taylor, D. (1939) Vitamin D in the treatment of arthritis. Can. Med. Assoc. J., 41, 601.
77 Guan, Y., Hao, Y., Guan, Y., Bu, H. & Wang, H. (2020) The effect of vitamin D supplementation on rheumatoid arthritis patients: a systematic review and meta-analysis. Front. Med., 7, 596 007.
78 Franco, A. S., Freitas, T. Q., Bernardo, W. M. & Pereira, R. M. R. (2017) Vitamin D supplementation and disease activity in patients with immune-mediated rheumatic diseases: A systematic review and meta-analysis. Medicine (Baltimore), 96, e7024.
79 Tang, T. et al. (2019) Adjunctive vitamin D for the treatment of active juvenile idiopathic arthritis: An open-label, prospective, randomized controlled trial. Exp. Ther. Med., 18, 4921–4926.
80 Mukherjee, D., Lahiry, S., Thakur, S. & Chakraborty, D. S. (2019) Effect of 1,25 dihydroxy vitamin D3 supplementation on pain relief in early rheumatoid arthritis. J. Fam. Med. Prim. Care, 8, 517–522.
81 Islam, M. A., Khandker, S. S., Alam, S. S., Kotyla, P. & Hassan, R. (2019) Vitamin D status in patients with systemic lupus erythematosus (SLE): A systematic review and meta-analysis. Autoimmun. Rev., 18, 102 392.
82 Zheng, R. et al. (2019) Efficacy and safety of vitamin D supplementation in patients with systemic lupus erythematosus: a meta-analysis of randomized controlled trials. Am. J. Med. Sci., 358, 104–114.
83 Dowling, G. B. & Prosser Thomas, E. W. (1946) Treatment of lupus vulgaris with calciferol. Lancet (London, England), 1, 919–922.
84 Lima, G. L., Paupitz, J. A., Aikawa, N. E., Alvarenga, J. C. & Pereira, R. M. R. (2018) A randomized double-blind placebo-controlled trial of vitamin D supplementation in juvenile-onset systemic lupus erythematosus: positive effect on trabecular microarchitecture using HR-pQCT. Osteoporos. Int., 29, 587–594.
85 Al-Kushi, A. G. et al. (2018) Effect of vitamin D and calcium supplementation in patients with systemic lupus erythematosus. Saudi J. Med. Med. Sci., 6, 137–142.
86 Wang, S., Wu, Y., Zuo, Z., Zhao, Y. & Wang, K. (2018) The effect of vitamin D supplementation on thyroid autoantibody levels in the treatment of autoimmune thyroiditis: a systematic review and a meta-analysis. Endocrine, 59, 499–505.
87 Behera, K. K. et al. (2020) Effect of vitamin D supplementation on thyroid autoimmunity among subjects of autoimmune thyroid disease in a coastal province of India: a randomized open-label trial. Niger. Med. J., 61, 237–240.
88 NICE (2020) Osteoporosis − prevention of fragility fractures. Health Topics A-Z, https://cks.nice.org.uk/topics/osteoporosis-prevention-of-fragility-fractures/.
89 Avenell, A., Mak, J. C. S. & O’Connell, D. (2014) Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst. Rev., 2014, CD000227.
90 Yao, P. et al. (2019) Vitamin D and calcium for the prevention of fracture: a systematic review and meta-analysis. JAMA Netw. Open, 2, e1917789.
91 Eleni, A. & Panagiotis, P. (2020) A systematic review and meta-analysis of vitamin D and calcium in preventing osteoporotic fractures. Clin. Rheumatol., doi:10.1007/s10067-020-05122-3.
92 Liu, C. et al. (2020) Effects of combined calcium and vitamin D supplementation on osteoporosis in postmenopausal women: a systematic review and meta-analysis of randomized controlled trials. Food Funct., 11, 10 817–10 827.
93 Hu, Z.-C. et al. (2019) Comparison of fracture risk using different supplemental doses of vitamin D, calcium or their combination: a network meta-analysis of randomised controlled trials. BMJ Open, 9, e024595.
94 Bolland, M. J., Grey, A. & Avenell, A. (2018) Effects of vitamin D supplementation on musculoskeletal health: a systematic review, meta-analysis, and trial sequential analysis. Lancet Diabetes Endocrinol., 6, 847–858.
95 Zhao, J.-G., Zeng, X.-T., Wang, J. & Liu, L. (2017) Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: a systematic review and meta-analysis. JAMA, 318, 2466–2482.
96 Silk, L. N., Greene, D. A. & Baker, M. K. (2015) The effect of calcium or calcium and vitamin D supplementation on bone mineral density in healthy males: a systematic review and meta-analysis. Int. J. Sport Nutr. Exerc. Metab., 25, 510–524.
97 Sluyter, J. D., Manson, J. E. & Scragg, R. (2021) Vitamin D and clinical cancer outcomes: a review of meta-analyses. JBMR Plus, 5, e10420.
98 Voutsadakis, I. A. (2021) Vitamin D baseline levels at diagnosis of breast cancer: A systematic review and meta-analysis. Hematol. Oncol. Stem Cell Ther., 14, 16–26.
99 Zhou, L., Chen, B., Sheng, L. & Turner, A. (2020) The effect of vitamin D supplementation on the risk of breast cancer: a trial sequential meta-analysis. Breast Cancer Res. Treat., 182, 1–8.
100 Shahvegharasl, Z. et al. (2020) Effects of cholecalciferol supplementation on serum angiogenic biomarkers in breast cancer patients treated with tamoxifen: A controlled randomized clinical trial. Nutrition, 72, 110 656.
101 Mohseni, H. et al. (2019) Genetic variations in VDR could modulate the efficacy of vitamin D3 supplementation on inflammatory markers and total antioxidant capacity among breast cancer women: a randomized double blind controlled trial. Asian Pac. J. Cancer Prev., 20, 2065–2072.
102 Going, C. C. et al. (2018) Vitamin D supplementation decreases serum 27-hydroxycholesterol in a pilot breast cancer trial. Breast Cancer Res. Treat., 167, 797–802.
103 Arnaout, A. et al. (2019) Randomized window of opportunity trial evaluating high-dose vitamin D in breast cancer patients. Breast Cancer Res. Treat., 178, 347–356.
104 Huang, D. et al. (2020) Additively protective effects of vitamin D and calcium against colorectal adenoma incidence, malignant transformation and progression: A systematic review and meta-analysis. Clin. Nutr., 39, 2525–2538.
105 Vaughan-Shaw, P. G. et al. (2020) The effect of vitamin D supplementation on survival in patients with colorectal cancer: systematic review and meta-analysis of randomised controlled trials. Br. J. Cancer, 123, 1705–1712.
106 Ng, K. et al. (2019) Effect of high-dose vs standard-dose vitamin D3 supplementation on progression-free survival among patients with advanced or metastatic colorectal cancer: the SUNSHINE randomized clinical trial. JAMA, 321, 1370–1379.
107 Yuan, C. & Ng, K. (2020) Vitamin D supplementation: a potential therapeutic agent for metastatic colorectal cancer. Br. J. Cancer, 123, 1205–1206.
108 Shahvazi, S., Soltani, S., Ahmadi, S. M., de Souza, R. J. & Salehi-Abargouei, A. (2019) The effect of vitamin D supplementation on prostate cancer: a systematic review and meta-analysis of clinical trials. Horm. Metab. Res., 51, 11–21.
109 Wagner, D. et al. (2013) Randomized clinical trial of vitamin D3 doses on prostatic vitamin D metabolite levels and Ki67 labeling in prostate cancer patients. J. Clin. Endocrinol. Metab., 98, 1498–1507.
110 Mahamat-Saleh, Y., Aune, D. & Schlesinger, S. (2020) 25-Hydroxyvitamin D status, vitamin D intake, and skin cancer risk: a systematic review and dose-response meta-analysis of prospective studies. Sci. Rep., 10, 13 151.
111 Li, X., He, J., Yu, M. & Sun, J. (2020) The efficacy of vitamin D therapy for patients with COPD: a meta-analysis of randomized controlled trials. Ann. Palliat. Med., 9, 286–297.
112 Chen, F.-Y., Xiao, M., Ling, B., Liu, L. & Chen, L. (2019) Vitamin D does not improve lung function decline in COPD: a meta-analysis. Eur. Rev. Med. Pharmacol. Sci., 23, 8637–8644.
113 Jolliffe, D. A. et al. (2019) Vitamin D to prevent exacerbations of COPD: systematic review and meta-analysis of individual participant data from randomised controlled trials. Thorax, 74, 337–345.
114 World Health Organization (2021) Cardiovascular disease (CVD). Factsheets, https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-%28cvds%29.
115 Nudy, M., Krakowski, G., Ghahramani, M., Ruzieh, M. & Foy, A. J. (2020) Vitamin D supplementation, cardiac events and stroke: A systematic review and meta-regression analysis. Int. J. Cardiol. Hear. Vasc., 28, 100 537.
116 Manson, J. E. et al. (2019) Vitamin D supplements and prevention of cancer and cardiovascular disease. N. Engl. J. Med., 380, 33–44.
117 Mirhosseini, N., Rainsbury, J. & Kimball, S. M. (2018) Vitamin D supplementation, serum 25(OH)D concentrations and cardiovascular disease risk factors: a systematic review and meta-analysis. Front. Cardiovasc. Med., 5, 87.
118 Wu, L. & Sun, D. (2017) Effects of calcium plus vitamin D supplementation on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J. Hum. Hypertens., 31, 547–554.
119 Farapti, F., Fadilla, C., Yogiswara, N. & Adriani, M. (2020) Effects of vitamin D supplementation on 25(OH)D concentrations and blood pressure in the elderly: a systematic review and meta-analysis. F1000Research, 9, 633.
120 He, S. & Hao, X. (2019) The effect of vitamin D3 on blood pressure in people with vitamin D deficiency: A system review and meta-analysis. Medicine (Baltimore), 98, e15284.
121 Shu, L. & Huang, K. (2018) Effect of vitamin D supplementation on blood pressure parameters in patients with vitamin D deficiency: a systematic review and meta-analysis. J. Am. Soc. Hypertens., 12, 488–496.
122 Morvaridzadeh, M. et al. (2020) Effect of calcium and vitamin D co-supplementation on blood pressure: a systematic review and meta-analysis. Clin. Ther., 42, e45–e63.
123 Dibaba, D. T. (2019) Effect of vitamin D supplementation on serum lipid profiles: a systematic review and meta-analysis. Nutr. Rev., 77, 890–902.
124 Ostadmohammadi, V. et al. (2019) The effects of vitamin D supplementation on glycemic control, lipid profiles and C-reactive protein among patients with cardiovascular disease: a systematic review and meta-analysis of randomized controlled trials. Curr. Pharm. Des., 25, 201–210.
125 Pincombe, N. L., Pearson, M. J., Smart, N. A., King, N. & Dieberg, G. (2019) Effect of vitamin D supplementation on endothelial function − An updated systematic review with meta-analysis and meta-regression. Nutr. Metab. Cardiovasc. Dis., 29, 1261–1272.
126 Mazidi, M., Karimi, E., Rezaie, P. & Vatanparast, H. (2017) The impact of vitamin D supplement intake on vascular endothelial function; a systematic review and meta-analysis of randomized controlled trials. Food Nutr. Res., 61, 1273574.
127 Hussin, A. M. et al. (2017) Effects of vitamin D supplementation on endothelial function: a systematic review and meta-analysis of randomised clinical trials. Eur. J. Nutr., 56, 1095–1104.
128 Cheng, Y.-C., Huang, Y.-C. & Huang, W.-L. (2020) The effect of vitamin D supplement on negative emotions: A systematic review and meta-analysis. Depress. Anxiety, 37, 549–564.
129 Jamilian, H. et al. (2019) The effects of vitamin D supplementation on mental health, and biomarkers of inflammation and oxidative stress in patients with psychiatric disorders: A systematic review and meta-analysis of randomized controlled trials. Prog. Neuropsychopharmacol. Biol. Psychiatry, 94, 109 651.
130 Lázaro Tomé, A. et al. (2021) Efficacy of vitamin D in the treatment of depression: a systematic review and meta-analysis. Actas Esp. Psiquiatr., 49, 12–23.
131 Zhu, C. et al. (2020) Vitamin D supplementation improves anxiety but not depression symptoms in patients with vitamin D deficiency. Brain Behav., 10, e01760.
132 Vellekkatt, F., Menon, V., Rajappa, M. & Sahoo, J. (2020) Effect of adjunctive single dose parenteral Vitamin D supplementation in major depressive disorder with concurrent vitamin D deficiency: A double-blind randomized placebo-controlled trial. J. Psychiatr. Res., 129, 250–256.
133 Kaviani, M., Nikooyeh, B., Zand, H., Yaghmaei, P. & Neyestani, T. R. (2020) Effects of vitamin D supplementation on depression and some involved neurotransmitters. J. Affect. Disord., 269, 28–35.
134 Krul-Poel, Y. H. M., Ter Wee, M. M., Lips, P. & Simsek, S. (2017) Management of endocrine disease: The effect of vitamin D supplementation on glycaemic control in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Eur. J. Endocrinol., 176, R1–R14.
135 Sahebi, R. et al. (2019) The effects of vitamin D supplementation on indices of glycemic control in Iranian diabetics: A systematic review and meta-analysis. Complement. Ther. Clin. Pract., 34, 294–304.
136 Li, X., Liu, Y., Zheng, Y., Wang, P. & Zhang, Y. (2018) The effect of vitamin D supplementation on glycemic control in type 2 diabetes patients: a systematic review and meta-analysis. Nutrients, 10, 375.
137 Wu, C., Qiu, S., Zhu, X. & Li, L. (2017) Vitamin D supplementation and glycemic control in type 2 diabetes patients: A systematic review and meta-analysis. Metabolism, 73, 67–76.
138 Lee, C. J. et al. (2017) The effect of vitamin D supplementation on glucose metabolism in type 2 diabetes mellitus: A systematic review and meta-analysis of intervention studies. J. Diabetes Complications, 31, 1115–1126.
139 Asbaghi, O., Khosroshahi, M. Z., Kashkooli, S. & Abbasnezhad, A. (2019) Effect of calcium‑vitamin D co‑supplementation on insulin, insulin sensitivity, and glycemia: a systematic review and meta-analysis of randomized clinical trials. Horm. Metab. Res., 51, 288–295.
140 Pramono, A., Jocken, J. W. E., Blaak, E. E. & van Baak, M. A. (2020) The effect of vitamin D supplementation on insulin sensitivity: a systematic review and meta-analysis. Diabetes Care, 43, 1659–1669.
141 Poolsup, N., Suksomboon, N. & Plordplong, N. (2016) Effect of vitamin D supplementation on insulin resistance and glycaemic control in prediabetes: a systematic review and meta-analysis. Diabet. Med., 33, 290–299.
142 He, S. et al. (2018) Effect of vitamin D supplementation on fasting plasma glucose, insulin resistance and prevention of type 2 diabetes mellitus in non-diabetics: A systematic review and meta-analysis. Biomed. Reports, 8, 475–484.
143 Zhang, Y. et al. (2020) Effects of vitamin D supplementation on prevention of type 2 diabetes in patients with prediabetes: a systematic review and meta-analysis. Diabetes Care, 43, 1650–1658.
144 Mansournia, M. A. et al. (2018) The effects of vitamin D supplementation on biomarkers of inflammation and oxidative stress in diabetic patients: a systematic review and meta-analysis of randomized controlled trials. Horm. Metab. Res., 50, 429–440.
145 Mousa, A., Naderpoor, N., Teede, H., Scragg, R. & de Courten, B. (2018) Vitamin D supplementation for improvement of chronic low-grade inflammation in patients with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Nutr. Rev., 76, 380–394.
146 Yu, Y., Tian, L., Xiao, Y., Huang, G. & Zhang, M. (2018) Effect of vitamin D supplementation on some inflammatory biomarkers in type 2 diabetes mellitus subjects: a systematic review and meta-analysis of randomized controlled trials. Ann. Nutr. Metab., 73, 62–73.
147 Han, Q., Li, X., Tan, Q., Shao, J. & Yi, M. (2019) Effects of vitamin D3 supplementation on serum 25(OH)D concentration and strength in athletes: a systematic review and meta-analysis of randomized controlled trials. J. Int. Soc. Sports Nutr., 16, 55.
148 Tomlinson, P. B., Joseph, C. & Angioi, M. (2015) Effects of vitamin D supplementation on upper and lower body muscle strength levels in healthy individuals. A systematic review with meta-analysis. J. Sci. Med. Sport, 18, 575–580.
149 Farrokhyar, F. et al. (2017) Effects of vitamin D supplementation on serum 25-hydroxyvitamin D concentrations and physical performance in athletes: a systematic review and meta-analysis of randomized controlled trials. Sports Med., 47, 2323–2339.
150 Abshirini, M., Mozaffari, H., Kord-Varkaneh, H., Omidian, M. & Kruger, M. C. (2020) The effects of vitamin D supplementation on muscle strength and mobility in postmenopausal women: a systematic review and meta-analysis of randomised controlled trials. J. Hum. Nutr. Diet., 33, 207–221.
151 Tabrizi, R. et al. (2019) The effects of vitamin D supplementation on muscle function among postmenopausal women: a systematic review and meta-analysis of randomized controlled trials. EXCLI J., 18, 591–603.
152 Rosendahl-Riise, H., Spielau, U., Ranhoff, A. H., Gudbrandsen, O. A. & Dierkes, J. (2017) Vitamin D supplementation and its influence on muscle strength and mobility in community-dwelling older persons: a systematic review and meta-analysis. J. Hum. Nutr. Diet., 30, 3–15.
153 Antoniak, A. E. & Greig, C. A. (2017) The effect of combined resistance exercise training and vitamin D(3) supplementation on musculoskeletal health and function in older adults: a systematic review and meta-analysis. BMJ Open, 7, e014619.
154 Wei, Y. et al. (2020) Effects of vitamin D supplementation in patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Int. J. Endocrinol. Metab., 18, e97205.
155 Tabrizi, R. et al. (2017) The effects of vitamin D supplementation on metabolic profiles and liver function in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis of randomized controlled trials. Diabetes Metab. Syndr., 11 Suppl 2, S975–S982.
156 Mansour-Ghanaei, F., Pourmasoumi, M., Hadi, A., Ramezani-Jolfaie, N. & Joukar, F. (2020) The efficacy of vitamin D supplementation against nonalcoholic fatty liver disease: a meta-analysis. J. Diet. Suppl., 17, 467–485.
157 Guo, X.-F. et al. (2020) Vitamin D and non-alcoholic fatty liver disease: a meta-analysis of randomized controlled trials. Food Funct., 11, 7389–7399.
158 Lukenda Zanko, V. et al. (2020) Vitamin D for treatment of non-alcoholic fatty liver disease detected by transient elastography: A randomized, double-blind, placebo-controlled trial. Diabetes. Obes. Metab., 22, 2097–2106.
159 Hussain, M. et al. (2019) Effect of vitamin D supplementation on various parameters in non-alcoholic fatty liver disease patients. Pak. J. Pharm. Sci., 32, 1343–1348.
160 Hales, C., Carroll, M., Fryar, C. & Ogden, C. (2020) Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief, no 360. https://www.cdc.gov/nchs/products/databriefs/db360.htm.
161 Hajhashemy, Z., Shahdadian, F., Ziaei, R. & Saneei, P. (2021) Serum vitamin D levels in relation to abdominal obesity: A systematic review and dose-response meta-analysis of epidemiologic studies. Obes. Rev., 22, e13134.
162 Perna, S. (2019) Is vitamin D supplementation useful for weight loss programs? A systematic review and meta-analysis of randomized controlled trials. Medicina (Kaunas), 55.
163 Golzarand, M., Hollis, B. W., Mirmiran, P., Wagner, C. L. & Shab-Bidar, S. (2018) Vitamin D supplementation and body fat mass: a systematic review and meta-analysis. Eur. J. Clin. Nutr., 72, 1345–1357.
164 Chandler, P. D. et al. (2015) Effect of vitamin D supplementation alone or with calcium on adiposity measures: a systematic review and meta-analysis of randomized controlled trials. Nutr. Rev., 73, 577–593.
165 Dinca, M. et al. (2016) Does vitamin D supplementation alter plasma adipokines concentrations? A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res., 107, 360–371.
166 Wu, Z., Malihi, Z., Stewart, A. W., Lawes, C. M. & Scragg, R. (2018) The association between vitamin D concentration and pain: a systematic review and meta-analysis. Public Health Nutr., 21, 2022–2037.
167 Zadro, J. R. et al. (2018) Is vitamin D supplementation effective for low back pain? A systematic review and meta-analysis. Pain Physician, 21, 121–145.
168 Gaikwad, M., Vanlint, S., Mittinity, M., Moseley, G. L. & Stocks, N. (2017) Does vitamin D supplementation alleviate chronic nonspecific musculoskeletal pain? A systematic review and meta-analysis. Clin. Rheumatol., 36, 1201–1208.
169 Yong, W. C., Sanguankeo, A. & Upala, S. (2017) Effect of vitamin D supplementation in chronic widespread pain: a systematic review and meta-analysis. Clin. Rheumatol., 36, 2825–2833.
170 Wu, Z., Malihi, Z., Stewart, A. W., Lawes, C. M. & Scragg, R. (2016) Effect of vitamin D supplementation on pain: a systematic review and meta-analysis. Pain Physician, 19, 415–427.
171 Polycystic ovary syndrome (2019) www.nhs.uk/conditions/ https://www.nhs.uk/conditions/polycystic-ovary-syndrome-pcos/.
172 Azadi-Yazdi, M., Nadjarzadeh, A., Khosravi-Boroujeni, H. & Salehi-Abargouei, A. (2017) The effect of vitamin D supplementation on the androgenic profile in patients with polycystic ovary syndrome: a systematic review and meta-analysis of clinical trials. Horm. Metab. Res., 49, 174–179.
173 Wang, L. et al. (2020) Effects of vitamin D supplementation on metabolic parameters of women with polycystic ovary syndrome: a meta-analysis of randomized controlled trials. Gynecol. Endocrinol., 1–10, doi:10.1080/09513590.2020.1813272.
174 Miao, C.-Y., Fang, X.-J., Chen, Y. & Zhang, Q. (2020) Effect of vitamin D supplementation on polycystic ovary syndrome: A meta-analysis. Exp. Ther. Med., 19, 2641–2649.
175 Łagowska, K., Bajerska, J. & Jamka, M. (2018) The role of vitamin D oral supplementation in insulin resistance in women with polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Nutrients, 10, 1637.
176 Akbari, M. et al. (2018) The effects of vitamin D supplementation on biomarkers of inflammation and oxidative stress among women with polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Horm. Metab. Res., 50, 271–279.
177 Fang, F. et al. (2017) Effect of vitamin D supplementation on polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Clin. Pract., 26, 53–60.
178 Lerchbaum, E. et al. (2021) Effects of vitamin D supplementation on surrogate markers of fertility in PCOS women: a randomized controlled trial. Nutrients, 13, 547.
179 Rasheedy, R., Sammour, H., Elkholy, A. & Salim, Y. (2020) The efficacy of vitamin D combined with clomiphene citrate in ovulation induction in overweight women with polycystic ovary syndrome: a double blind, randomized clinical trial. Endocrine, 69, 393–401.
180 Zhuang, L., Cui, W., Cong, J. & Zhang, Y. (2019) Efficacy of vitamin D combined with metformin and clomiphene in the treatment of patients with polycystic ovary syndrome combined with infertility. Iran. J. Public Health, 48, 1802–1809.
181 Gallo, S. et al. (2020) Vitamin D supplementation during pregnancy: an evidence analysis center systematic review and meta-analysis. J. Acad. Nutr. Diet., 120, 898-924.e4.
182 Jahanjoo, F., Farshbaf-Khalili, A., Shakouri, S. K. & Dolatkhah, N. (2018) Maternal and neonatal metabolic outcomes of vitamin D supplementation in gestational diabetes mellitus: a systematic review and meta-analysis. Ann. Nutr. Metab., 73, 145–159.
183 Wang, M. et al. (2020) The effects of vitamin D supplementation on glycemic control and maternal-neonatal outcomes in women with established gestational diabetes mellitus: A systematic review and meta-analysis. Clin. Nutr., doi:10.1016/j.clnu.2020.12.016.
184 Keller, A. et al. (2020) The role of vitamin D in the development of diabetes post gestational diabetes mellitus: a systematic literature review. Nutrients, 12, 1733.
185 Ojo, O., Weldon, S. M., Thompson, T. & Vargo, E. J. (2019) The effect of vitamin D supplementation on glycaemic control in women with gestational diabetes mellitus: a systematic review and meta-analysis of randomised controlled trials. Int. J. Environ. Res. Public Health, 16, 1716.
186 Rodrigues, M. R. K. et al. (2019) Efficacy of vitamin D supplementation in gestational diabetes mellitus: Systematic review and meta-analysis of randomized trials. PLoS One, 14, e0213006.
187 Akbari, M. et al. (2017) The effects of vitamin D supplementation on glucose metabolism and lipid profiles in patients with gestational diabetes: a systematic review and meta-analysis of randomized controlled trials. Horm. Metab. Res., 49, 647–653.
188 Aguilar-Cordero, M. J. et al. (2020) Vitamin D, preeclampsia and prematurity: A systematic review and meta-analysis of observational and interventional studies. Midwifery, 87, 102 707.
189 Fogacci, S. et al. (2020) Vitamin D supplementation and incident preeclampsia: A systematic review and meta-analysis of randomized clinical trials. Clin. Nutr., 39, 1742–1752.
190 Khaing, W. et al. (2017) Calcium and vitamin D supplementation for prevention of preeclampsia: a systematic review and network meta-analysis. Nutrients, 9, 1141.
191 Maugeri, A., Barchitta, M., Blanco, I. & Agodi, A. (2019) Effects of vitamin D supplementation during pregnancy on birth size: a systematic review and meta-analysis of randomized controlled trials. Nutrients, 11, 442.
192 Bi, W. G. et al. (2018) Association between vitamin D supplementation during pregnancy and offspring growth, morbidity, and mortality: a systematic review and meta-analysis. JAMA Pediatr., 172, 635–645.
193 Martineau, A. R. et al. (2019) Vitamin D supplementation to prevent acute respiratory infections: individual participant data meta-analysis. Health Tech. Assess. (Winchester, England), 23, 1–44.
194 Jolliffe, D. A. et al. (2021) Vitamin D supplementation to prevent acute respiratory infections: a systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol., doi:10.1016/S2213-8587(21)00051-6.
195 Teshome, A., Adane, A., Girma, B. & Mekonnen, Z. A. (2021) The impact of vitamin D level on COVID-19 infection: systematic review and meta-analysis. Front. Public Health, 9, 624 559.
196 Liu, N. et al. (2021) Low vitamin D status is associated with coronavirus disease 2019 outcomes: a systematic review and meta-analysis. Int. J. Infect. Dis., 104, 58–64.
197 Kazemi, A. et al. (2021) Association of vitamin D status with SARS-CoV-2 infection or COVID-19 severity: a systematic review and meta-analysis. Adv. Nutr., doi:10.1093/advances/nmab012.
198 Petrelli, F. et al. (2021) Therapeutic and prognostic role of vitamin D for COVID-19 infection: A systematic review and meta-analysis of 43 observational studies. J. Steroid Biochem. Mol. Biol., 211, 105 883.
199 Pereira, M., Dantas Damascena, A., Galvão Azevedo, L. M., de Almeida Oliveira, T. & da Mota Santana, J. (2020) Vitamin D deficiency aggravates COVID-19: systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr., 1–9, doi:10.1080/10408398.2020.1841090.
200 Rastogi, A. et al. (2020) Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study). Postgrad. Med. J., doi:10.1136/postgradmedj-2020-139065.
201 Murai, I. H. et al. (2021) Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. JAMA, 325, 1053–1060.
202 Entrenas Castillo, M. et al. (2020) Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J. Steroid Biochem. Mol. Biol., 203, 105 751.
203 Giannini, S. et al. (2021) Effectiveness of in-hospital cholecalciferol use on clinical outcomes in comorbid COVID-19 patients: a hypothesis-generating study. Nutrients, 13, 219.
204 Annweiler, G. et al. (2020) Vitamin D supplementation associated to better survival in hospitalized frail elderly COVID-19 patients: the GERIA-COVID quasi-experimental study. Nutrients, 12, 3377.
205 Annweiler, C. et al. (2020) Vitamin D and survival in COVID-19 patients: A quasi-experimental study. J. Steroid Biochem. Mol. Biol., 204, 105 771.
206 Ohaegbulam, K. C., Swalih, M., Patel, P., Smith, M. A. & Perrin, R. (2020) Vitamin D supplementation in COVID-19 patients: a clinical case series. Am. J. Ther., 27, e485–e490.
207 Griffin, G. et al. (2020) Vitamin D and COVID-19: evidence and recommendations for supplementation. R. Soc. Open Sci., 7, 201 912.
208 Malihi, Z., Wu, Z., Mm Lawes, C. & Scragg, R. (2017) Noncalcemic adverse effects and withdrawals in randomized controlled trials of long-term vitamin D2 or D3 supplementation: a systematic review and meta-analysis. Nutr. Rev., 75, 1007–1034.
209 Vitamin D (2021) naturalmedicines.therapeuticresearch.com https://naturalmedicines.therapeuticresearch.com/databases/food,-herbs-supplements/professional.aspx?productid=929.
210 Holick, M. F. et al., Endocrine Society (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab., 96(7), 1911−1930.