By Karin Elgar
Magnesium is a co-factor for more than 300 different enzymatic processes, and therefore plays a role in virtually every process in the cell, including cellular energy production, neuromuscular and cardiac function, maintaining ionic gradients, regulation of cell membrane receptors and DNA, RNA and protein synthesis. It is also an essential structural component for DNA and RNA on the cellular level, as well as in bones and teeth.
Whilst overt magnesium deficiency is rare, subclinical deficiency appears to be common, and increases the risk of many chronic conditions. Organic magnesium formulations, such as citrate, have been shown to be slightly better absorbed than inorganic ones, but many clinical trials have used inorganic formulations of magnesium, mostly oxide and chloride, and have shown benefits in a range of conditions, including cardiometabolic conditions, bone health, pain and constipation.
Cite as: Elgar K. Magnesium: A Review of Clinical Use and Efficacy. Nutr Med J., 1 (1): 79-99.
Affiliation: K. Elgar is with the Nutritional Medicine Institute, London, UK.
Corresponding author: Karin Elgar (email info@karinelgar.com)
Article history: Received 10 March 2021. Peer-reviewed and received in revised form 26 May 2021. Accepted 27 May 2021. Available online 15 July 2021.
Published by: The Nutritional Medicine Institute
Open Access: This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs licence (http:// creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reproduction and distribution of the work, in any medium, provided the original work is not altered or transformed in any way, and that the work is properly cited. For commercial use please contact support@nmi.health
Introduction
Magnesium is involved in hundreds of essential physiological processes, and adequate levels are therefore vital for general health. Whilst overt hypomagnesaemia appears to be relatively rare, suboptimal levels and inadequate dietary intakes are common, and may lead to an increased risk of chronic disease.
Magnesium deficiency can arise from poor diet, malabsorption, kidney disease, alcoholism and drug interactions. Various tests are in use to determine magnesium levels; however, none of them is considered particularly accurate. Serum levels are commonly used and, whilst low serum levels can accurately reflect magnesium deficiency, normal serum levels do not exclude low-grade magnesium deficiency. A combination of serum magnesium, urinary excretion and dietary intake measures appears to be the most practical and accurate way to determine magnesium status.
Good food sources include green leafy vegetables, cacao, wholegrains, nuts and seeds. Various dietary components, including certain types of fibre, phytates and oxalates can hinder absorption, which needs to be taken into account. In food supplements, organic formulations appear to be better absorbed than inorganic ones, and absorption is also better with several small as opposed to one large dose.
The most common side-effect of magnesium at high doses is diarrhoea. Caution is advised in patients with kidney disease as renal excretion plays an important part in magnesium homeostasis. Magnesium can also interact with a number of pharmaceutical drugs.
Functions
Magnesium is the fourth most abundant cation in our bodies (after sodium, potassium and calcium), and the second most common intracellular cation (after potassium).1 Magnesium is a co-factor for more than 300 different enzymatic processes, and therefore plays a role in virtually every process in the cell.2,3
Magnesium is an essential co-factor for various enzymes involved in glycolysis and the Krebs cycle,3 and mitochondrial magnesium also appears to play an important part in regulating mitochondrial function.4 As such, magnesium is essential for cellular energy production.
Magnesium acts as an antagonist to calcium, for example in neuromuscular and cardiac function, and helps maintain ionic gradients, i.e. keeping intracellular sodium and calcium low and potassium high.5 In the nervous system, magnesium is also involved in the regulation of various cell membrane receptors, including N-methyl-D-aspartate (NMDA) and ƴ-aminobutyric acid (GABA) receptors.3
Magnesium is involved in DNA, RNA and protein synthesis, and is an essential component in DNA and RNA structure.3 Magnesium also serves as a structural component in bones and teeth.
Deficiency
About 60% of magnesium is contained in the bones, another 30% in muscles. Less than 1% of total body magnesium is extracellular, i.e. in serum or plasma, and serum levels are maintained within a narrow range through homeostatic mechanisms, mainly renal excretion/reabsorption and gastrointestinal absorption of magnesium. When necessary, magnesium can also be released from bones and muscles to maintain serum levels when intake is low. Therefore, serum magnesium levels are not reflective of total body magnesium status, and normal serum levels do not necessarily rule out magnesium deficiency.6
A number of tests are used in research and in clinic to determine magnesium status; however, their accuracy and reliability is controversial,6,7 which impacts on the interpretation and comparability of study results.
- Retention of an intravenous (IV) magnesium load (administering IV magnesium followed by 24-hour urine collection) is considered the most reliable test, but is invasive and not practical for routine measurement. This test is also not reliable in individuals with impaired kidney function.
- Bone or muscle magnesium content through biopsy. Again, this is not practical for routine measurements.
- Urinary excretion.
- Red blood cell (RBC) magnesium. This test is often considered.
- The most commonly used test is serum magnesium levels; however, as mentioned above, this test is not reliable to identify mild magnesium deficiency as serum magnesium levels are tightly controlled and will therefore only drop in severe deficiency.
- Dietary intake of magnesium is commonly used to assess magnesium status. It is estimated that 30−50% of dietary magnesium is absorbed, but this depends on other nutrients. High intakes of fibre, phytates and oxalates can reduce magnesium absorption.8
Some authors suggest that a combination of serum magnesium, urinary excretion and dietary intake appears to be the most practical and accurate way to determine magnesium status.7
In clinical practice, RBC magnesium is commonly considered to be more accurate than serum level testing. Studies in animals support this method;9 however, studies in humans are contradictory, with some finding RBC magnesium levels to be superior to serum levels in determining low magnesium status,10 whilst others find it to be no more accurate.11 When choosing tests in clinical practice, it is important to bear such contradictory findings in mind, and also to take into account dietary history and symptoms.
The definition of hypomagnesaemia (low serum levels of magnesium) varies according to the source, but is commonly described as serum magnesium levels of less than 0.7 mmol/l12 although, more recently, BMJ Best Practice considered levels of below 0.9 mmol/l to constitute hypomagnesaemia.13 It is generally agreed that hypomagnesaemia is indicative of low total body magnesium levels, but that serum levels can remain stable despite low total body magnesium stores due to the various regulatory mechanisms described above.
Acute symptoms of hypomagnesaemia do not usually become apparent until serum levels drop below 0.5 mmol/l.14 As hypomagnesaemia is commonly associated with other metabolic abnormalities, in particular, hypokalaemia, hypocalcaemia and metabolic acidosis, it is difficult to determine which symptoms are solely due to the hypomagnesaemia.15 Anorexia, nausea, vomiting, lethargy and weakness are typical early symptoms of magnesium deficiency.15 Symptoms can be grouped into the following.
- Neuromuscular:
- Muscular weakness, apathy, tremors, paraesthesia, tetany, vertical nystagmus and positive Chvostek’s and Trousseau’s signs.
- Seizures, drowsiness, confusion and coma occur at magnesium concentrations below 0.4 mmol/l.
- Cardiovascular:
- Various electrocardiogram changes.
- Atrial and ventricular arrhythmias.
- Metabolic:
- Hypokalaemia, hypocalcaemia, metabolic acidosis.
Whilst overt magnesium deficiency is rare, magnesium insufficiency appears to be common and may have significant implications for long-term health. Longer-term complications of magnesium deficiency that commonly go unrecognised include the following.2,14
- Altered glucose metabolism;
- Metabolic syndrome;
- Hypertension;
- Atherosclerosis;
- Osteoporosis;
- Asthma;
- Migraines;
- Pre-eclampsia;
- Cardiovascular disease (CVD).
These will be discussed in detail below in Clinical uses.
Possible causes of hypomagnesaemia include the following.15
- Low dietary intake:
- Malnutrition, including anorexia nervosa.
- Malabsorption:
- Coeliac disease;
- Inflammatory bowel disease;
- Chronic diarrhoea;
- Steatorrhoea;
- Short bowel syndrome.
- Parathyroid disorders.
- Chronic alcoholism.
- Various medications, including:
- Proton pump inhibitors (PPIs);16
- Diuretics, including loop and thiazide diuretics;
- Some antimicrobials, including Amphotericin B, aminoglycosides, Foscarnet;
- Chemotherapy drugs, such as Cisplatin;
- Immunosuppressants, such as Tacrolimus, cyclosporine.
Prevalence estimates for hypomagnesaemia are thought to be in the range of 2.5−15% of the general population, but as it is often asymptomatic it may be commonly underdiagnosed.17 The largest prevalence study, conducted with over 16,000 unselected subjects in Germany, found a prevalence of 14.5%.18 Interestingly, the authors used a definition of hypomagnesaemia of < 0.76 mmol/l, and noted that prevalence would only be 2% if they used a more conservative cut-off point of < 0.7 mmol/l. Prevalence appears to be significantly higher in specific populations: 65% of patients in intensive care units; and 30% in a study on subjects admitted for alcoholism.17
Food Sources
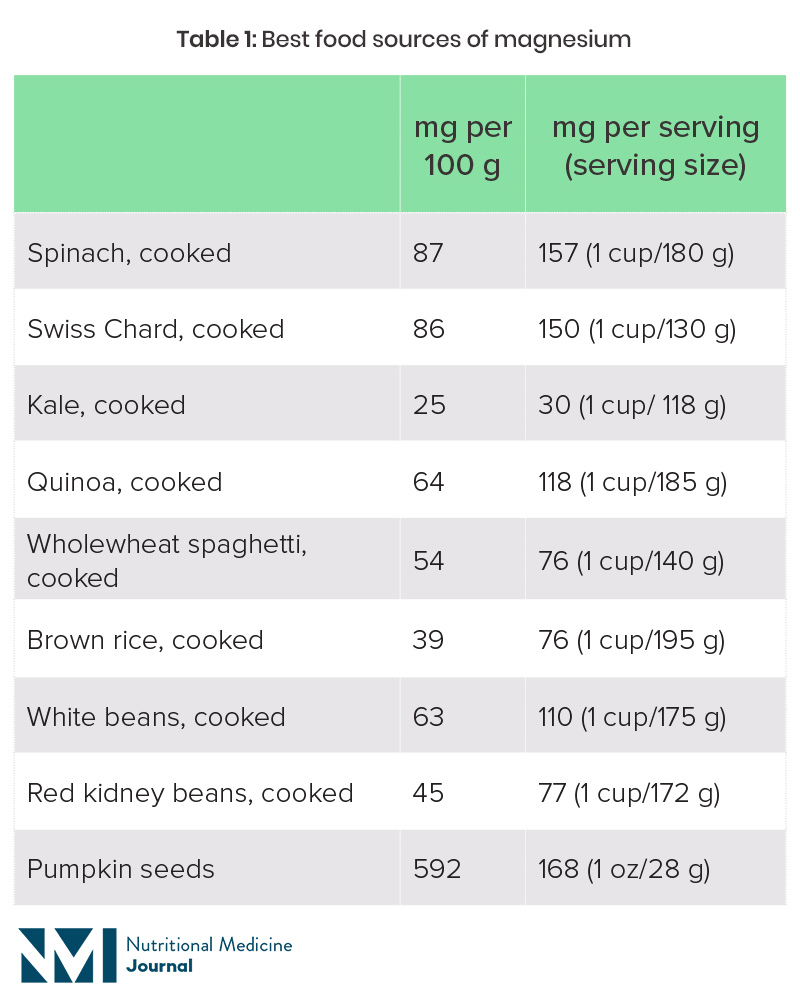
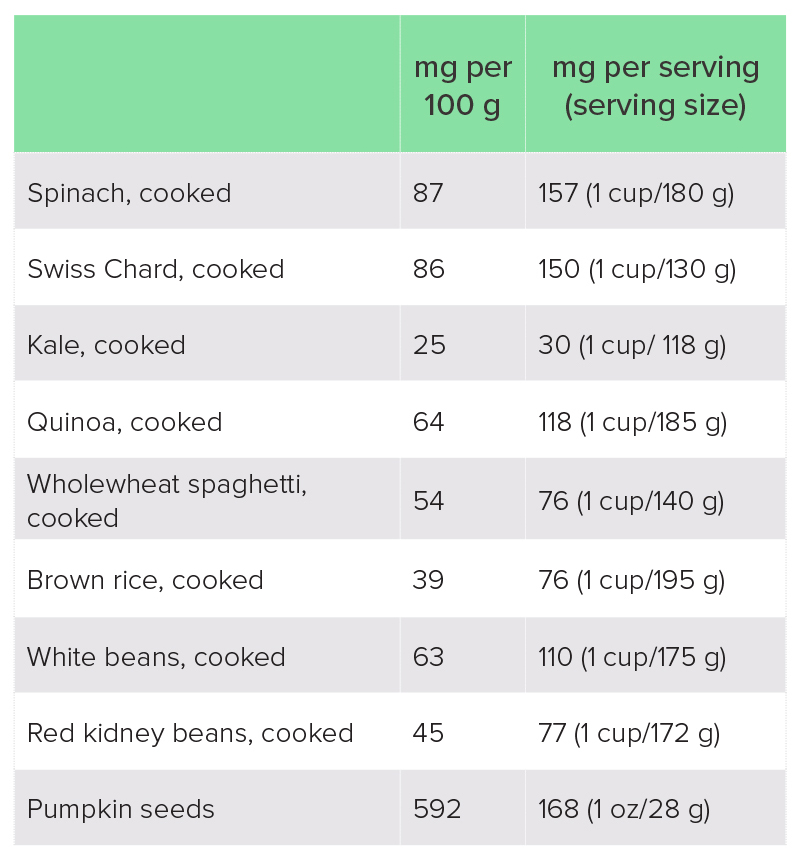
Magnesium is part of the chlorophyll molecule in plants, which is what gives plants their green colour. Green leafy vegetables are therefore one of the best sources of dietary magnesium. Nuts and seeds, pulses and wholegrains are other good sources, although these foods are also high in phytates, which hinder magnesium absorption. Refined and highly processed foods tend to be depleted of magnesium.
Table 1: Best food sources of magnesium19
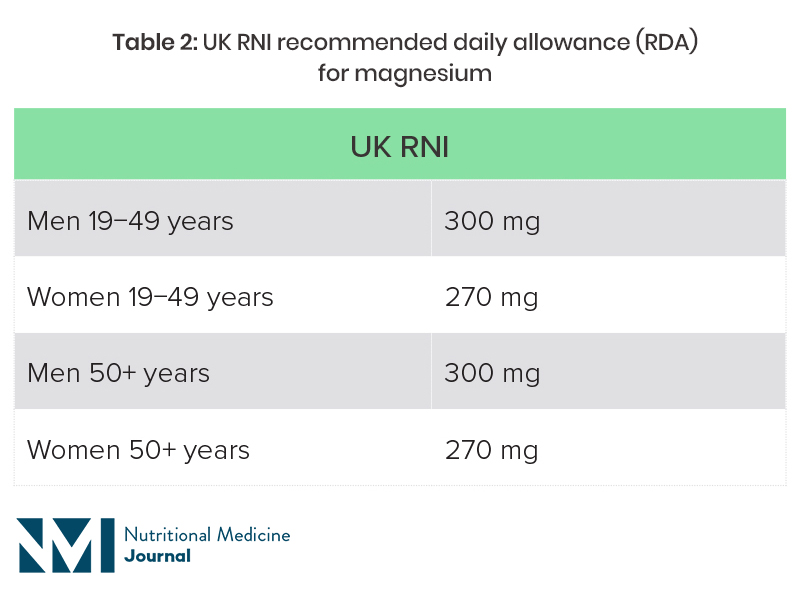
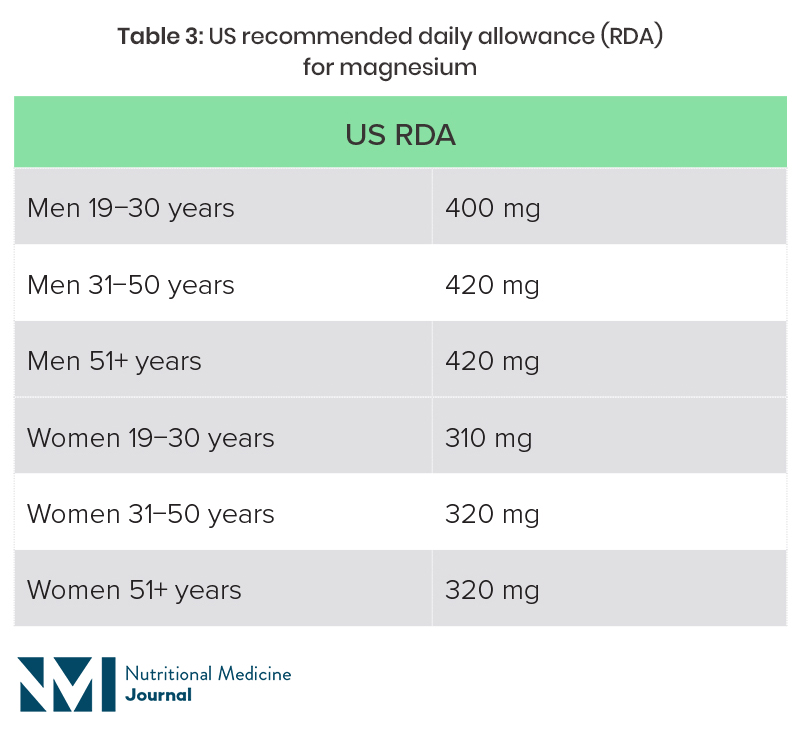
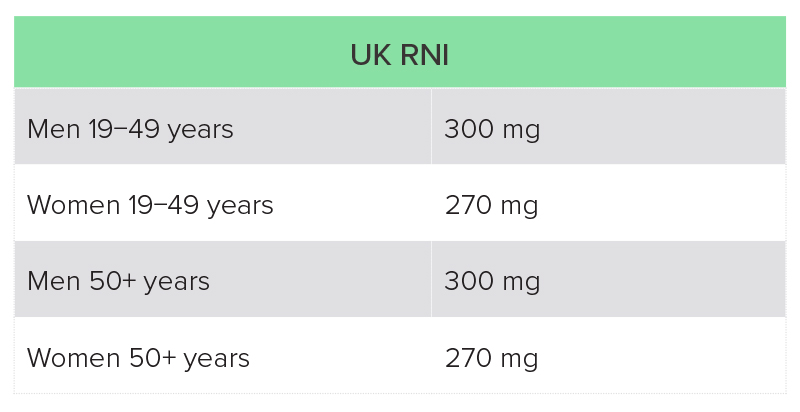
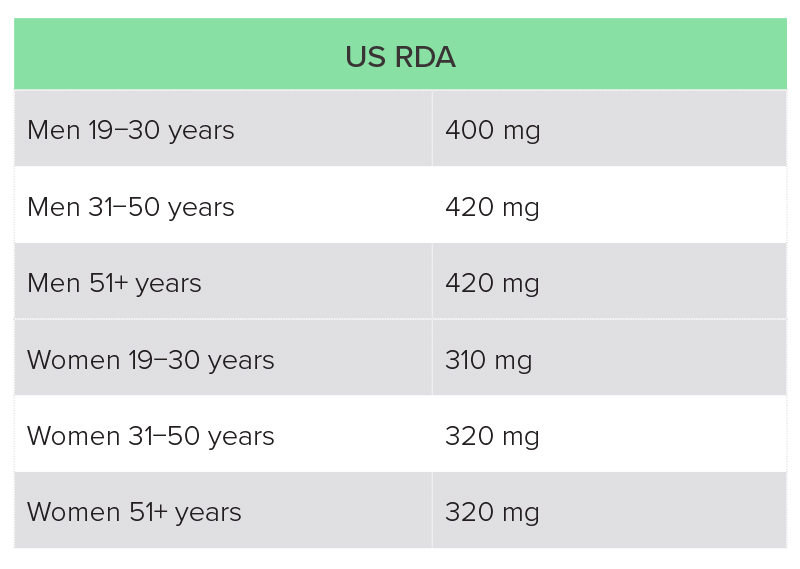
The recommended magnesium intake varies from country to country, and is also dependent on age and sex (Table 2).20,21 Dietary surveys in both the UK and USA suggest that a high proportion of individuals are not getting the recommended intake through diet.
US National Health and Nutrition Examination Survey (NHANES) 2013−2016: average intake for adult men was 344 mg magnesium per day, and for women 270 mg, with 55% and 51% of adult men and women, respectively, not getting the recommended intake from food (fortified and non-fortified) and water alone.22
UK National Diet and Nutrition Survey (NDNS): average intake of magnesium from food was 302 mg for men aged 19−65 years, and 238 mg for women in this age group, which is below the reference nutrient intake (RNI)a for women. Fourteen percent of men and 11% of women were getting less than the lower reference nutrient intake (LRNI), which is 190 mg for men and 150 mg for women. The average intake in those aged over 65 years was 242 mg, with 16% having a magnesium intake of less than the LRNI.23
Table 2: UK RNI and US recommended daily allowance (RDA) for magnesium
RNI, reference nutrient intake.
RDA, recommended daily allowance.
Supplements
Magnesium is mostly absorbed via both saturable and non-saturable active transport pathways in the small intestine; smaller amounts are absorbed in the colon. Magnesium is absorbed as Mg2+ ions. Absorption rates depend on the magnesium status of the person, and are usually between 30% and 50%, but can be as high as 80% and as low as 20%.24
Due to serum magnesium levels being tightly controlled within a narrow range, absorption and bioavailability studies are fraught with methodological difficulties, which may explain the sometimes conflicting results. Overall, it appears that organic magnesium formulations are slightly better absorbed than inorganic ones.24 In particular, a number of studies found magnesium citrate to be better absorbed than magnesium oxide.25−27 Despite these findings, many clinical trials have used inorganic formulations of magnesium, mostly oxide and chloride, and have shown benefits in a range of conditions (see below, Clinical uses).
Other factors play important roles in magnesium absorption, for example:
Dietary factors:28 high doses of other minerals, partly fermentable fibres (e.g. hemicellulose), non-fermentable fibres (e.g. cellulose, lignin), phytates and oxalates hinder, whilst protein, medium-chain-triglycerides, resistant starch, oligosaccharides, inulin, mannitol and lactulose enhance absorption.
Magnesium dose:29 several small doses are better absorbed than one large dose.
Transdermal magnesium has a long tradition of use, such as in Epsom salt (magnesium sulphate) and Dead Sea salt (which is high in magnesium) baths. However, good-quality evidence that magnesium is absorbed transdermally is lacking. A small safety study on a magnesium-containing barrier cream showed no significant increase in serum magnesium levels after 4 days of repeated administration.30 A couple of small trials suggest that magnesium can be absorbed through the skin. A small pilot study suggests that a magnesium cream may increase serum magnesium levels over the course of 2 weeks.31 Another small pilot study found that a magnesium chloride spray significantly increased magnesium levels in six out of nine subjects over 12 weeks by using hair mineral analysis to evaluate magnesium levels.32 It should be noted that hair mineral analysis is not a generally recognised and standardised method of determining magnesium status, and that the evidence base for benefits of magnesium in clinical use (see Clinical uses) has been built on oral, and to a lesser extent IV, supplementation, rather than transdermal.
Clinical Uses
Metabolic syndrome
The term metabolic syndrome refers to a cluster of signs and symptoms, including insulin resistance, obesity, abnormal blood lipids and hypertension, which increase the risk of developing CVD and diabetes. The exact definition has changed over the years, and varies depending on the organisation.33
Epidemiological studies have shown consistently that metabolic syndrome is associated with low magnesium levels,34−36 and that those with low intakes of magnesium are at increased risk of developing metabolic syndrome.36−38
A 2016 review of 27 double-blind clinical trials investigating the effects of supplementation with magnesium on components of metabolic syndrome (insulin resistance, hyperglycaemia, hypertension, dyslipidaemia) found that all six of the trials that looked at insulin resistance showed an improvement in the Homeostatic Model Assessment for Insulin Resistance (HOMA-IR).39 Results were mixed, with some studies showing improvements for hyperglycaemia, hypertension, triglycerides and low-density lipoprotein (LDL)-cholesterol, but others not.39 The types of studies included in this review varied considerably with regards to study population, formulation and dose of magnesium, length of intervention and baseline magnesium levels.
Only one of the 27 studies included in the review mentioned above39 investigated the effects of magnesium in patients with metabolic syndrome.40 This double-blind, placebo-controlled trial found no significant improvements in any of the laboratory parameters tested in the magnesium (400 mg of magnesium chelate per day for 12 weeks) versus placebo group. Interestingly, 23% of participants were found to have hypomagnesaemia, and 36% had signs of cellular magnesium depletion at baseline, and magnesium status did not improve with supplementation.40 The authors of this study hypothesise that insulin resistance, which was present in the majority of the patients, may have impaired magnesium entrance into the cells, and suggest that higher dosages may be necessary for patients with insulin resistance.
Two more recent double-blind, placebo-controlled studies in patients with metabolic syndrome and hypomagnesaemia at baseline found that magnesium supplementation improved not only magnesium status but also insulin sensitivity in both trials,41,42 as well as blood pressure (BP) and triglycerides in one of the trials.42 The studies used magnesium oxide solution (385 mg elemental magnesium per day for 3 months)41 and magnesium chloride solution (382 mg magnesium per day for 16 weeks),42 respectively.
Another double-blind, placebo-controlled study in overweight, insulin-resistant but non-diabetic individuals with normal magnesium levels showed that 6 months of supplementation with magnesium aspartate hydrochloride, 365 mg per day, improved insulin sensitivity.43
The evidence overall supports the use of magnesium in patients with metabolic syndrome, in particular with regards to improving insulin sensitivity. Dosages used in clinical trials have ranged largely from 300 to 450 mg per day, and the most commonly used formulations were magnesium chloride, oxide and aspartate.
Type 2 diabetes mellitus
There has been a significant increase in T2DM over the past decades. In 2018, it was estimated that 13% of all US adults had diabetes, with 90−95% of these having T2DM,44,45 whilst in the UK the prevalence of diabetes was estimated to be 7% in 2018/2019.46 Both low dietary magnesium intake and low serum magnesium levels have been shown in epidemiological studies to increase the risk of developing T2DM,47−51 as well as increase the risk of complications in those with T2DM due to poor glycaemic control.52,53
In 2017, a systematic review and meta-analysis of 28 randomised controlled trials (RCTs) including 1694 diabetic subjects found that magnesium supplementation improved a number of cardiovascular risk factors, including fasting glucose, high-density lipoprotein (HDL)- and LDL-cholesterol, triglycerides and diastolic BP. The authors noted that effects were more pronounced in patients with low magnesium levels.54 For example, one RCT showed that 360 mg magnesium (as lactate) for 3 months did not change glycaemic control or blood lipids in diabetics with normal serum magnesium levels, ‘normal’ defined as > 0.74 mmol/l. It is noteworthy that mean serum magnesium levels at baseline were 0.92 mmol/l, which might be considered ‘optimal’.55
A recent double-blind, placebo-controlled clinical trial showed that supplementing with 250 mg magnesium per day (as oxide) for 24 weeks improved glycaemic control, LDL- and total cholesterol, and carotid intima-media thickness, in diabetics receiving haemodialysis.56 In another more recent randomised trial, 250 mg magnesium per day (as oxide, gluconate, lactate) improved glycaemic control in patients with T2DM after 3 months of supplementation.57
Magnesium has also been investigated as a supplement alongside other nutrients: 250 mg magnesium per day (as oxide) for 4 months as part of a comprehensive multivitamin and mineral supplement improved neuropathic symptoms, but not glycaemic control, capillary blood flow or electrophysiological measures in diabetics.58 Concomitant supplementation of magnesium, 500 mg per day as oxide, and choline has been shown to improve inflammation, endothelial factors and coagulation biomarkers.59,60 Meanwhile, supplementation with a honey fortified with magnesium, cinnamon and chromium led to a significant reduction in total and LDL-cholesterol, but no improvements in glycaemic control. The dose of magnesium in this trial was low: 120 mg per day (as citrate).61
The evidence shows that diabetics can benefit from magnesium supplementation in terms of improved glycaemic control and cardiovascular risk factors, especially in patients who are low in magnesium. Dosages used in studies showing benefits have generally ranged from 250 to 638 mg per day in a variety of formulations.
Hypertension
Magnesium is important for relaxation of the smooth muscles of the vascular system as well as other functions that are important in the regulation of BP.6 Two meta-analyses of prospective cohort studies showed that BP was inversely related to magnesium levels and intake.49,62 A number of cross-sectional studies also found an inverse relationship between magnesium intake and/or levels and BP,63−68 although some cross-sectional studies do not confirm an association.69
A meta-analysis of 34 double-blind, placebo-controlled trials assessing the effectiveness of magnesium supplementation to lower BP in both subjects with and without hypertension showed a significant benefit of magnesium supplementation, and suggested that a dose of 300 mg elemental magnesium for 1 month was sufficient to increase serum magnesium levels and lower BP.70 There was significant heterogeneity within the study results that may be explained by the theory that certain subsets of patients may benefit more than others. A meta-analysis of RCTs including only hypertensive patients with a systolic BP of > 155 mmHg and who had previously used antihypertensive drugs showed a particularly strong effect of magnesium supplementation, with a mean decrease in systolic BP of 18.7 mmHg and in diastolic BP of 10.9 mmHg.71 A 2017 meta-analysis of 11 RCTs found that magnesium supplementation significantly lowered both systolic and diastolic BP in a subset of patients with prediabetes, insulin resistance or other chronic conditions.72
The evidence suggests a significant BP-lowering effect of magnesium, especially in those with hypertension or other chronic conditions, at a dose of 300 mg per day.
Cardiovascular disease
A 2013 meta-analysis of 19 prospective cohort studies concluded that both dietary magnesium intake and serum magnesium levels were inversely related to CVD risk, including coronary heart disease, death from CVD and stroke.73 However, a more recent meta-analysis of 40 prospective cohort studies found a benefit of higher magnesium intakes only for heart failure and stroke, but not total CVD. It also found a lower risk of all-cause mortality in those with higher magnesium intakes.74
Atherosclerosis
Magnesium deficiency can cause calcification of soft tissues, and may promote the development and progression of atherosclerosis.6 A number of epidemiological studies have shown an inverse relationship of atherosclerosis with serum magnesium levels75−78 and dietary magnesium intake79 in patients with both high and low cardiovascular risk.
A case series of 80 patients reports that a combination of injected and oral magnesium improved (non-vascular) soft tissue calcification in 75% of patients.80 Also, a 6-month double-blind magnesium supplementation study showed that magnesium chelate, 600 mg per day, not only lowered BP, but also improved endothelial function and attenuated subclinical atherosclerosis in hypertensive women on magnesium-depleting anti-hypertensive medication.81
Although the evidence for benefits of magnesium in atherosclerosis is limited, the fact that magnesium also appears to improve many other cardiovascular risk factors suggests that recommending magnesium supplementation to patients with atherosclerosis seems warranted, at a dose dependent on other risk factors present.
Osteoporosis
Osteoporosis is characterised by a loss of bone mass that leads to a weakening of the bone and an increased risk of fractures. As mentioned above, magnesium forms a structural part of bones, and bones also serve as a reservoir for magnesium.6
Magnesium is intricately involved with vitamin D metabolism: magnesium is necessary for the synthesis and conversion of vitamin D, whilst vitamin D is also important for magnesium absorption.82 Magnesium has been used to reverse vitamin D-resistant rickets.83
Epidemiological studies have shown lower bone mass density and an increased incidence of osteoporosis in people with low magnesium levels84 and low magnesium intakes in both adults85−87 and adolescents.88 A meta-analysis of seven case−control studies including 1349 women showed that low magnesium levels are a risk factor for osteoporosis, although there were regional/racial differences.89 Both high and low magnesium levels and intakes have shown harmful effects on bone health,90 although the evidence is inconsistent.91
Evidence for the benefits of magnesium in bone health from supplementation studies is limited. Two short-term (30 days), open-label, controlled magnesium supplementation trials showed that magnesium decreased markers of bone turnover in healthy young men,92 as well as in post-menopausal women.93 The daily dosages were 365 mg (as carbonate and oxide) and 1830 mg magnesium citrate (it is not stated in the article whether this refers to elemental weight), respectively. A 12-month double-blind, placebo-controlled study on magnesium supplementation (300 mg as magnesium oxide) in children and adolescents with low magnesium intake showed an increase in bone mineral content.94 An open-label, controlled trial of post-menopausal women with osteoporosis showed that magnesium supplementation (250−750 mg per day as magnesium hydroxide) for 2 years significantly improved bone density. Serum magnesium levels significantly increased during magnesium supplementation in this study.95
Based on the clinical evidence available, magnesium appears to be of benefit for bone health in children and adolescents as well as in healthy adults and post-menopausal women at a dosage of 300 mg per day in children and 250−750 mg per day in adults.
Asthma
Lower magnesium levels have been reported in asthmatics, but results from epidemiological studies are heterogenous. A recent metanalysis found an increased susceptibility for asthma with low magnesium levels only for Asians, whilst Caucasian and African population-pooled results did not show statistical significance.96 There is evidence that in patients with asthma, the condition appears to be less well controlled in those with low magnesium levels,97−99 although other studies did not find an association between magnesium levels and severity of asthma.100,101 It is noteworthy that individuals in the latter two studies had magnesium levels within the normal range, whilst in the former studies asthmatics with worse pulmonary function/poorer control were considered to have magnesium levels below normal range, suggesting that asthmatics with low levels of magnesium are more likely to benefit from supplementation.
There is a reasonably large body of evidence for the use of IV magnesium sulphate in acute asthma attacks as an adjunct to other treatments, including bronchodilators and corticosteroids. The evidence is more consistent for children102,103 than for adults.104,105 Nebulised, inhaled magnesium sulphate has also been used in acute asthma exacerbations, alongside other treatments, but results from clinical trials are inconsistent, and systematic reviews and meta-analyses on the whole do not find enough evidence for significant benefits.102,106,107
A recent meta-analysis of seven RCTs evaluated the benefits of oral supplementation of magnesium in mild to moderate asthmatics. Whilst there were improvements in all outcome parameters, they were only statistically significant for one parameter, which led the authors to the conclusion that at present there is not enough evidence to recommend oral magnesium supplementation for asthmatics.108 The authors point out that there was significant heterogeneity amongst the studies with regards to dose, formulation and length of treatment, and no sub-group analysis was performed to potentially identify those patients for whom magnesium supplementation may be of benefit. Looking at the studies individually, five out of the seven studies found a benefit of oral magnesium supplementation, possibly more so in those studies where magnesium levels were found to be low at baseline.109−111
Although the evidence for use of oral magnesium for asthma is mixed, overall it suggests a benefit, especially in those patients with low magnesium levels at baseline. Dosages in studies showing benefits have ranged from 200 to 300 mg per day in children and adolescents, and 340 to 450 mg per day in adults.
Pain
A systematic review and meta-analysis of 27 RCTs supports the use of IV magnesium sulphate as an adjunct to anaesthesia in reducing pain scores and analgesia use post-operatively.112 Oral magnesium lozenges have also been shown to reduce post-operative sore throat.113
Two double-blind, placebo-controlled trials looked at the benefits of magnesium in neuropathic pain, one of which showed that IV magnesium, followed by oral magnesium, 500 mg per day, significantly reduced pain scores and increased motility in patients with chronic lower back pain.114 However, another double-blind, placebo-controlled trial failed to show a significant benefit of magnesium, 330 mg as chloride per day, over placebo in neuropathic pain, although there was a significant improvement with regards to the frequency of pain paroxysms (attacks) and emotional impact in the magnesium group.115 It is noteworthy that in the latter trial there were significant improvements in neuropathic pain in both the magnesium and the placebo groups.
A 2001 Cochrane review and meta-analysis based on three RCTs concluded that magnesium was more effective than placebo to provide pain relief in dysmenorrhoea.116 A recent RCT in women with dysmenorrhoea also showed that IV magnesium alongside opium and buprenorphine reduced pain and improved quality of life.117
Magnesium levels tend to be lower in migraine sufferers, and there are a number of possible mechanisms by which magnesium may be involved in the pathogenesis of migraines.118 A 2016 review and meta-analysis evaluated the use of magnesium in migraines, and found IV magnesium to be efficacious in acute attacks, and oral magnesium to reduce both frequency and intensity of migraines.119 These findings have been confirmed by more recent clinical trials, one in adults, one in children, using 1000 mg per day (as oxide) and 75−375 mg per day depending on body weight (as oxide or glycinate), respectively.120,121
An interesting series of case studies also shows potential for high-dose oral magnesium to alleviate the pain caused by erythromelalgia,122 a rare condition characterised by episodes of burning pain, usually in the extremities, and it is mentioned as a treatment option on the NHS website.123
Magnesium appears to be of benefit in a number of painful conditions, including neuropathic pain, dysmenorrhoea and migraines, with dosages used ranging from 500 to 1000 mg per day in adults, and 75 to 375 mg per day in children.
Fibromyalgia syndrome
Fibromyalgia syndrome is a complex pain syndrome that is also associated with insomnia, irritable bowel syndrome and other symptoms, and there is some overlap with CFS. There is limited evidence for the effectiveness of magnesium supplementation in FMS.
Two cross-sectional studies did not find a correlation between serum magnesium levels and FMS, but patients with FMS tend to have lower dietary magnesium intake.124,125 An intervention study found women with FMS to have lower serum and erythrocyte magnesium levels, and compared the effectiveness of treatments with magnesium, 300 mg per day as citrate, amitriptyline, and magnesium plus amitriptyline. Whilst magnesium reduced some symptoms, amitriptyline on its own reduced almost all symptoms, with the combination being most effective.126
A double-blind, placebo-controlled trial of magnesium with malic acid showed no benefits during the 4-week blinded phase with a low dose of 150 mg magnesium and 600 mg malic acid, but showed significant benefits in the severity of pain and tenderness scores in an open-label extension of the study after 2 and 6 months, with a dose in excess of 400 mg magnesium and 1600 mg malic acid.127
Whilst the evidence is limited, magnesium at a dose of at least 300 mg per day could be trialled
in patients with FMS.
Chronic fatigue syndrome
Mitochondrial dysfunction is thought to be an important factor in CFS, and magnesium is therefore commonly used in clinical practice in patients with CFS.
An early clinical trial found magnesium levels to be lower in people with CFS, and weekly magnesium sulphate injections to improve symptoms of pain, energy and emotional state.128 However, no further research appears to have confirmed these promising results.129
Pre-eclampsia and pregnancy-induced hypertension (PIH)
Pre-eclampsia is a complication of pregnancy characterised by high BP, proteinuria and oedema. It can progress to eclampsia, which is a significant cause for perinatal and maternal morbidity and mortality. IV or intramuscular magnesium is recommended for women with pre-eclampsia by the World Health Organisation.130
Epidemiological studies suggest that women affected by pre-eclampsia or PIH have lower levels of magnesium.131−134 However, a Cochrane review and meta-analysis in 2014 found no statistically significant reduction in pre-eclampsia with magnesium supplementation.130 The authors pointed out that only two of the 10 studies reviewed were of high quality. A more recent RCT including 180 pregnant women showed that magnesium supplementation (200 mg per day) reduced the incidence of a number of pregnancy complications, including pre-eclampsia.135
The currently available evidence for benefits of magnesium to prevent pre-eclampsia is weak.
Depression
Two reviews and meta-analyses suggest an inverse relationship between magnesium levels or intake and the risk of depression.136,137 This association has been confirmed in two more recent studies,138,139 whilst in another study an association did not reach statistical significance.140
Whether oral magnesium supplementation is of benefit in patients with depression appears to depend on the dose and/or whether patients are magnesium-deficient: a 2018 double-blind, placebo-controlled trial showed no benefit of magnesium alongside fluoxetine.141 Magnesium dosage was low (120 mg) compared with other clinical trials. A trial in post-partum depression did not find a benefit of magnesium at a dose of 64.5 mg (elemental, as sulphate).142 On the other hand, 500 mg of magnesium (as oxide) was shown in a double-blind, placebo-controlled study to decrease depression scores in depressed patients with low magnesium levels.143 Another RCT showed that 450 mg of magnesium (as chloride) was as effective as the tricyclic antidepressant imipramine in treating depression in elderly type 2 diabetics with hypomagnesaemia.
Although the evidence is mixed, at a dose of 450−500 mg per day, magnesium appears to be of benefit in patients with depression who have low magnesium levels.
Anxiety
The authors of a 2017 review on magnesium and anxiety concluded that magnesium may have a positive effect on mild to moderate anxiety, although they state that the current evidence base is poor. Only one of the eight trials reviewed in this article looked at magnesium on its own, the other studies included other botanicals or vitamin B6.144
Pre-menstrual syndrome (PMS)
The above review144 also looked at the effects of magnesium on anxiety as a symptom of PMS, and found that four out of the seven relevant studies showed a positive effect of magnesium either on its own or in combination with vitamin B6, but also noted a number of methodological issues with the clinical trials reviewed.
A review and meta-analysis of observational studies looking at magnesium levels and PMS showed no significant association, although there was significant heterogeneity amongst the 13 studies reviewed; in particular, studies conducted outside of USA showed an inverse relationship between magnesium levels and PMS.145
A double-blind trial comparing magnesium, 250 mg per day (formulation not reported), magnesium plus vitamin B6, and placebo demonstrated that magnesium plus vitamin B6 was the most and placebo the least effective in relieving PMS symptoms.146 The same research group also compared magnesium, 250 mg per day (formulation not reported), and vitamin B6 alone against placebo in subjects with PMS in another double-blind trial, and found a significant improvement in PMS scores in all three groups but more so in both the magnesium only and the vitamin B6 only groups than with placebo.147 An open-label RCT also found significant benefits of magnesium, 250 mg per day as a modified-release formulation, in women with PMS but, due to lack of a placebo, results have to be interpreted with caution as there appears to be a significant placebo effect in PMS.148
Clinical research of magnesium for PMS is limited but promising, with a dose of 250 mg per day showing benefits.
Sleep
Lower intakes of magnesium are associated with shorter sleep time,149 and magnesium appears to be involved in regulating circadian rhythms.150 A small study in infants showed that serum magnesium levels were associated with sleep behaviour.151
However, evidence from clinical trials for the use of magnesium supplementation to improve sleep is limited. A double-blind, placebo-controlled study in elderly subjects with primary insomnia found that supplementing 500 mg magnesium (as oxide) improved both subjective and objective sleep parameters.152 Baseline dietary magnesium intakes were low in this patient group. Another double-blind, placebo-controlled trial of magnesium, 225 mg per day (formulation not mentioned), in combination with melatonin and zinc also showed benefits in elderly subjects with insomnia.153
Although clinical research into magnesium and sleep is limited, a dose of 225−500 mg magnesium has been shown to improve sleep.
RLS/periodic limb movement in sleep (PLMS)
Low magnesium levels are associated with an increased incidence of PLMS,154 and anecdotal evidence suggests magnesium supplementation to be of benefit.155 However, there is only one open pilot trial published that reports benefits of oral magnesium, 301 mg per day (formulation not mentioned), for RLS and PLMS.156 A double-blind, placebo-controlled trial of 28 patients, which formed the basis of a doctoral thesis, failed to show significant benefits of magnesium in RLS.157
At present there is insufficient evidence to show a benefit of magnesium for RLS.
Muscle cramps
Two reviews and meta-analyses found that magnesium did not help with leg cramps in the general population, but may have a small effect in pregnant women, although the evidence was considered weak.158,159 A more recent trial on magnesium for nocturnal leg cramps in elderly subjects also found magnesium, 520 mg per day (as oxide), to have no benefits over placebo, although there was a strong placebo effect.160
The currently available evidence suggests that magnesium has no benefit for leg cramps.
Constipation
Magnesium salts act as osmotic laxatives,161 and are used for both bowel clearance in preparation for procedures such as colonoscopies and in the management of chronic constipation. Low intake of magnesium has been shown to be associated with an increased risk of constipation independent of fibre intake.162
Magnesium and sulphate-rich mineral water has been shown in a number of double-blind, placebo-controlled clinical trials to be beneficial in chronic/functional constipation.163−166 A recent double-blind, placebo-controlled trial also reports good results with supplementation of magnesium oxide (1.5 g per day, equivalent to 915 mg elemental magnesium) in patients with functional constipation.167 Another trial in elderly patients with constipation showed benefits of supplementation with magnesium hydroxide.168
Magnesium salts have also been shown to help with constipation in children and infants in a number of clinical trials, both on their own and in combination with other compounds.169−172
Prolonged use of high doses of magnesium, as commonly used in constipation, can lead to hypermagnesaemia, especially in those with kidney disorders, and monitoring of serum magnesium levels is advised.173,174
Whilst there is good evidence for the use of magnesium in constipation, the dosages needed are high and could cause harm with prolonged use.
Dyspepsia
Magnesium salts, in particular magnesium hydroxide, carbonate and trisilicate, are commonly used in antacids, either on their own or in combination with aluminium salts and/or alginates (which coats the surface of the stomach). The magnesium salts can help relieve symptoms of indigestion, gastro-oesophageal reflux, gastritis and gastric ulcers. They tend to work quickly, but are not recommended for long-term use as they do not deal with the underlying cause, and can have side-effects and drug interactions.175−177
Dose Recommendations
The mineral content of fruit and vegetables has declined significantly over past decades,178,179 and processing of foods further reduces their magnesium content. Surveys show that significant proportions of the population are not getting adequate levels of magnesium through their diet (see above). Supplementing magnesium on a daily basis to reduce the risk of developing chronic disease appears to be prudent. The Linus Pauling Institute, Oregon State University, USA, recommends a supplemental dose of 100 mg per day, alongside a magnesium-rich diet.180 In view of the likely shortfall, this seems to be a reasonable dose for preventive purposes.
Clinical studies that looked at therapeutic use of magnesium in various conditions have used a range of dosages, but the most commonly used doses were between 300 and 400 mg per day.
Safety
The most common side-effects of excessive magnesium intake are gastrointestinal disturbances, in particular diarrhoea. Hypermagnesaemia, defined as a serum magnesium level above 2.6 mg/dl, is rare and is most commonly seen in patients with kidney disease. Caution is therefore advised for the use of magnesium supplements in patients with kidney disease. Excessive use of magnesium, usually as a laxative or antacid, can also cause hypermagnesaemia at intakes over 2500 mg magnesium (elemental). Symptoms include weakness, hypotension, respiratory depression, and can lead to cardiac arrest.181−183
Magnesium from food does not induce diarrhoea or other side-effects. The upper tolerable limit (UTL) of magnesium from supplements is based on the amount that does not induce diarrhoea, and is in addition to intakes from food.
The European Food Safety Agency (EFSA) set a UTL for magnesium of 250 mg for adults, including pregnant and lactating women, and children from 4 years.183
The US Food and Nutrition Board, Institute for Medicine, set a UTL for magnesium of 350 mg for adults, including pregnant and lactating women, and children from 9 years, 110 mg for children aged 4−8 years, and 65 mg for children aged 1−3 years.21
Magnesium supplements can interact with a number of medications:21
Bisphosphonates (drugs to treat osteoporosis): magnesium can decrease absorption. Magnesium should be administered at least 2 hours apart.
Tetracycline and quinolone antibiotics: these can form complexes with magnesium, and should therefore be administered at least 2 hours before or 4−6 hours after the magnesium supplement.
Diuretics: loop and thiazide diuretics, including furosemide and bumetanide, hydrochlorothiazide and ethacrynic acid, can deplete magnesium and lead to hypomagnesaemia. Potassium-sparing diuretics, such as amiloride and spironolactone, on the other hand, also have a magnesium-sparing effect and can therefore raise magnesium levels.
PPIs: long-term use of PPIs can lead to hypomagnesaemia, and magnesium levels should be monitored in such patients.
Levodopa/carbidopa: a recent study showed that magnesium oxide may significantly reduce absorption of levodopa/carbidopa.184
Digoxin: magnesium-based antacids interfere with the absorption of digoxin.185
Sulphonylureas (type of anti-diabetic drug): magnesium-based antacids alter intestinal pH, and can therefore lead to an increased absorption of sulphonylurea drugs, potentially leading to hypoglycaemia.186,187
Conclusion
Magnesium has been shown to be of benefit in a range of conditions, including diabetes, hypertension, metabolic syndrome, pain and for bone health. The most commonly used dosages in clinical trials have been in the range of 300-400 mg per day, which is generally considered safe. The most common side effect with an excessive dose is diarrhoea. Caution should be exercised in patients with kidney disease and magnesium can interact with a number of medications.
Acknowledgments
Author contributions: K. Elgar carried out the literature review and formulated the manuscript.
Peer-reviewers and editors: the Nutritional Medicine Institute thanks the peer-reviewers
and editors for their important contributions.
Funding: Open Access publication was supported by an unrestricted donation from Pure Encapsulations, Sudbury, MA, USA. No other funding or sponsorship has been received for this work.
Declaration of interest: K. Elgar has received consultancy fees from Pure Encapsulations, Sudbury, MA, USA. This article is the independent work of the author and Pure Encapsulations was not involved in the decision to publish this research.
Bibliography
1 Wester, P. (1987) Magnesium. Am. J. Clin. Nutr., 45, 1305–1312.
2 Saris, N. E. L., Mervaala, E., Karppanen, H., Khawaja, J. A. & Lewenstam, A. (2000) Magnesium: An update on physiological, clinical and analytical aspects. Clin. Chim. Acta, 294, 1–26.
3 de Baaij, J. H. F., Hoenderop, J. G. J. & Bindels, R. J. M. (2015) Magnesium in man: Implications for health and disease. Physiol. Rev., 95, 1–46.
4 Panov, A. & Scarpa, A. (1996) Mg2+ control of respiration in isolated rat liver mitochondria. Biochemistry, 35, 12,849–12,856.
5 Al-Ghamdi, S. M. G., Cameron, E. C. & Sutton, R. A. L. (1994) Magnesium deficiency: Pathophysiologic and clinical overview. Am. J. Kidney Dis., 24, 737–752.
6 DiNicolantonio, J. J., O’Keefe, J. H. & Wilson, W. (2018) Subclinical magnesium deficiency: A principal driver of cardiovascular disease and a public health crisis. Open Hear., 5, e000668.
7 Costello, R. B. & Nielsen, F. (2017) Interpreting magnesium status to enhance clinical care: Key indicators. Curr. Opin. Clin. Nutr. Metab. Care, 20, 504–511.
8 Rude, R. K. (1998) Magnesium deficiency: A cause of heterogenous disease in humans. J. Bone Miner. Res., 13, 749–758.
9 Xiong, W., Liang, Y., Li, X., Liu, G. & Wang, Z. (2019) A direct quantitative analysis of erythrocyte intracellular ionized magnesium in physiological and pathological conditions. Biol. Pharm. Bull., 42, 357–364.
10 Simşek, E., Karabay, M. & Kocabay, K. (2005) Assessment of magnesium status in newly diagnosed diabetic children: measurement of erythrocyte magnesium level and magnesium tolerance testing. Turk. J. Pediatr., 47, 132–137.
11 Arnold, A., Tovey, J., Mangat, P., Penny, W. & Jacobs, S. (1995) Magnesium deficiency in critically ill patients. Anaesthesia, 50, 203–205.
12 (2013) Hypomagnesaemia. Drug Ther. Bull., 51, 33–36.
13 Romani, A. (2019) Evaluation of magnesium deficiency.
14 Pham, P. C. T. et al. (2014) Hypomagnesemia: A clinical perspective. Int. J. Nephrol. Renovasc. Dis., 7, 219–230
15 Ayuk, J. & Gittoes, N. J. L. (2014) Contemporary view of the clinical relevance of magnesium homeostasis. Ann. Clin. Biochem., 51, 179–188.
16 Luk, C. P., Parsons, R., Lee, Y. P. & Hughes, J. D. (2013) Hipomagnesemia asociada a inhibidores de la bomba de protones: ¿qué nos dicen los datos de la administración de alimentos y medicamentos? Ann. Pharmacother., 47, 773–780.
17 Mouw, D. R., Latessa, R. A. & Sullo, E. J. (2005) What are the causes of hypomagnesemia? J. Fam. Pract. 54, 174–175.
18 Schimatschek, H. F. & Rempis, R. (2001) Prevalence of hypomagnesemia in an unselected German population of 16,000 individuals. Magnes. Res., 14, 283–290.
19 U.S. Department of Agriculture, A. R. S. (2019) No Title. FoodData Central fdc.nal.usda.gov.
20 BNF (2016) Nutrition requirements. Br. Nutr. Found., 1–9.
21 IOM, Institute of Medicine (1997) Dietary Reference Intakes: Calcium, Phosphorus, Magnesium, Vitamin D and Fluoride (National Academy Press).
22 Moshfegh, A. J., Goldman, J. D., Rhodes, D. G., Clemens, J. C. & LaComb, R. (2019) Usual nutrient intake from food and beverages: What we eat in America. NHANES, 2013−2016.
23 Public Health England (2018) Statistical Summary: National Diet and Nutrition Survey: results from Years 7 and 8 (combined) of the Rolling Programme. Public Heal. England, London, UK, 8, 1–5.
24 Schuchardt, J. P. & Hahn, A. (2017) Intestinal absorption and factors influencing bioavailability of magnesium−an update. Curr. Nutr. Food Sci., 13, 260–278.
25 Lindberg, J. S., Zobitz, M. M., Poindexter, J. R. & Pak, C. Y. (1990) Magnesium bioavailability from magnesium citrate and magnesium oxide. J. Am. Coll. Nutr., 9, 48–55.
26 Walker, A. F., Marakis, G., Christie, S. & Byng, M. (2003) Mg citrate found more bioavailable than other Mg preparations in a randomised, double-blind study. Magnes. Res., 16, 183–191.
27 Kappeler, D. et al. (2017) Higher bioavailability of magnesium citrate as compared to magnesium oxide shown by evaluation of urinary excretion and serum levels after single-dose administration in a randomized cross-over study. BMC Nutr., 3, 7.
28 Bohn, T. (2008) Current nutrition & food science. Curr. Nutr. Food Sci., 4, 1–20
29 Fine, K. D., Santa Ana, C. A., Jack L. Porter, A. & Fordtran, J. S. (1991) Intestinal absorption of magnesium from food and supplements. J. Clin. Invest., 88, 396–402.
30 Eisenkraft, A. et al. (2009) Phase I study of a topical skin protectant against chemical warfare agents. Mil. Med., 174, 47–52
31 Kass, L. et al. (2017) Effect of transdermal magnesium cream on serum and urinary magnesium levels in humans: A pilot study. PLoS One, 12, e0174817
32 Watkins, K. & Josling, P. (2010). A pilot study to determine the impact of transdermal magnesium treatment on serum levels and whole body CaMg ratios. Nutr. Pract., 14, 1–7.
33 Huang, P. L. (2009) A comprehensive definition for metabolic syndrome. Dis. Model. Mech., 2, 231–237.
34 Wang, Y. et al. (2018) Association between serum magnesium concentration and metabolic syndrome, diabetes, hypertension and hyperuricaemia in knee osteoarthritis: a cross-sectional study in Hunan Province, China. BMJ Open, 8, e019159.
35 La, S. A. et al. (2016) Low magnesium levels in adults with metabolic syndrome: a meta-analysis. Biol. Trace Elem. Res., 170, 33–42.
36 Sarrafzadegan, N., Khosravi-Boroujeni, H., Lotfizadeh, M., Pourmogaddas, A. & Salehi-Abargouei, A. (2016) Magnesium status and the metabolic syndrome: A systematic review and meta-analysis. Nutrition, 32, 409–417.
37 Dibaba, D. T., Xun, P., Fly, A. D., Yokota, K. & He, K. (2014) Dietary magnesium intake and risk of metabolic syndrome: a meta-analysis. Diabet. Med., 31, 1301–1309.
38 Ju, S.-Y., Choi, W.-S., Ock, S.-M., Kim, C.-M. & Kim, D.-H. (2014) Dietary magnesium intake and metabolic syndrome in the adult population: dose-response meta-analysis and meta-regression. Nutrients, 6, 6005–6019.
39 Guerrero-Romero, F., Jaquez-Chairez, F. O. & Rodriguez-Moran, M. (2016) Magnesium in metabolic syndrome: a review based on randomized, double-blind clinical trials. Magnes. Res., 29, 146–153.
40 Lima de Souza E Silva, M. de L. et al. (2014) Magnesium replacement does not improve insulin resistance in patients with metabolic syndrome: a 12-week randomized double-blind study. J. Clin. Med. Res., 6, 456–462.
41 Toprak, O. et al. (2017) Magnesium replacement improves the metabolic profile in obese and pre-diabetic patients with mild-to-moderate chronic kidney disease: A 3-month, randomised, double-blind, placebo-controlled study. Kidney Blood Press. Res., 42, 33–42
42 Rodriguez-Moran, M., Simental-Mendia, L. E., Gamboa-Gomez, C. I. & Guerrero-Romero, F. (2018) Oral magnesium supplementation and metabolic syndrome: A randomized double-blind placebo-controlled clinical trial. Adv. Chronic Kidney Dis., 25, 261–266.
43 Mooren, F. C. et al. (2011) Oral magnesium supplementation reduces insulin resistance in non-diabetic subjects − a double-blind, placebo-controlled, randomized trial. Diabetes Obes. Metab., 13, 281–284.
44 Centres for Disease Control and Prevention (2020) National Diabetes Statistics Report, 2020. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf.
45 Centres for Disease Control and Prevention (2019) Type 2 Diabetes. https://www.cdc.gov/diabetes/basics/type2.html
46 Diabetes Prevalence 2019 (2020) diabetes.org.uk https://www.diabetes.org.uk/professionals/position-statements-reports/statistics/diabetes-prevalence-2019
47 Spiga, R. et al. (2019) Are circulating Mg(2+) levels associated with glucose tolerance profiles and incident type 2 diabetes? Nutrients, 11, 2460.
48 Zhao, B. et al. (2019) Association of magnesium consumption with type 2 diabetes and glucose metabolism: a systematic literature review and pooled study with trial sequential analysis. Diabetes Metab. Res. Rev., e3243, doi:10.1002/dmrr.3243.
49 Wu, J., Xun, P., Tang, Q., Cai, W. & He, K. (2017) Circulating magnesium levels and incidence of coronary heart diseases, hypertension, and type 2 diabetes mellitus: a meta-analysis of prospective cohort studies. Nutr. J., 16, 60.
50 Schutten, J. C. et al. (2019) Lower plasma magnesium, measured by nuclear magnetic resonance spectroscopy, is associated with increased risk of developing type 2 diabetes mellitus in women: Results from a Dutch prospective cohort study. J. Clin. Med., 8, 169.
51 Xu, T., Chen, G. C., Zhai, L. & Ke, K. F. (2015) Nonlinear reduction in risk for type 2 diabetes by magnesium intake: An updated meta-analysis of prospective cohort studies. Biomed. Environ. Sci., 28, 527–534.
52 Joy, S. S., George, T. P. & Siddiqui, K. (2019) Low magnesium level as an indicator of poor glycemic control in type 2 diabetic patients with complications. Diabetes Metab. Syndr., 13, 1303–1307.
53 Kumar, P., Bhargava, S., Agarwal, P. K., Garg, A. & Khosla, A. (2019) Association of serum magnesium with type 2 diabetes mellitus and diabetic retinopathy. J. Fam. Med. Prim. Care, 8, 1671–1677.
54 Verma, H. & Garg, R. (2017) Effect of magnesium supplementation on type 2 diabetes associated cardiovascular risk factors: a systematic review and meta-analysis. J. Hum. Nutr. Diet., 30, 621–633.
55 Navarrete-Cortes, A. et al. (2014) No effect of magnesium supplementation on metabolic control and insulin sensitivity in type 2 diabetic patients with normomagnesemia. Magnes. Res., 27, 48–56.
56 Talari, H. R. et al. (2019) Effects of magnesium supplementation on carotid intima-media thickness and metabolic profiles in diabetic haemodialysis patients: a randomised, double-blind, placebo-controlled trial. Br. J. Nutr., 121, 809–817.
57 ELDerawi, W. A., Naser, I. A., Taleb, M. H. & Abutair, A. S. (2018) The effects of oral magnesium supplementation on glycemic response among type 2 diabetes patients. Nutrients, 11, 44.
58 Farvid, M. S., Homayouni, F., Amiri, Z. & Adelmanesh, F. (2011) Improving neuropathy scores in type 2 diabetic patients using micronutrients supplementation. Diabetes Res. Clin. Pract., 93, 86–94.
59 Rashvand, S., Mobasseri, M. & Tarighat-Esfanjani, A. (2019) Effects of choline and magnesium concurrent supplementation on coagulation and lipid profile in patients with type 2 diabetes mellitus: A pilot clinical trial. Biol. Trace Elem. Res., doi:10.1007/s12011-019-01802-7.
60 Rashvand, S., Mobasseri, M. & Tarighat-Esfanjani, A. (2019) The effects of choline and magnesium co-supplementation on metabolic parameters, inflammation, and endothelial dysfunction in patients with type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled trial. J. Am. Coll. Nutr., 38, 714–721.
61 Whitfield, P., Parry-Strong, A., Walsh, E., Weatherall, M. & Krebs, J. D. (2016) The effect of a cinnamon-, chromium- and magnesium-formulated honey on glycaemic control, weight loss and lipid parameters in type 2 diabetes: an open-label cross-over randomised controlled trial. Eur. J. Nutr., 55, 1123–1131.
62 Han, H. et al. (2017) Dose-response relationship between dietary magnesium intake, serum magnesium concentration and risk of hypertension: a systematic review and meta-analysis of prospective cohort studies. Nutr. J., 16, 26.
63 Bain, L. K. M. et al. (2015) The relationship between dietary magnesium intake, stroke and its major risk factors, blood pressure and cholesterol, in the EPIC-Norfolk cohort. Int. J. Cardiol., 196, 108–114.
64 Choi, M.-K. & Bae, Y. J. (2015) Association of magnesium intake with high blood pressure in Korean adults: Korea National Health and Nutrition Examination Survey 2007−2009. PLoS One, 10, e0130405.
65 Chidambaram, N. et al. (2014) Relationship of sodium and magnesium intakes to hypertension proven by 24-hour urianalysis in a South Indian population. J. Clin. Hypertens. (Greenwich), 16, 581–586.
66 Singh, R. B. et al. (1996) Epidemiological study of magnesium status and risk of hypertension in a rural population of north India. Magnes. Res., 9, 173–181.
67 Ma, J. et al. (1995) Associations of serum and dietary magnesium with cardiovascular disease, hypertension, diabetes, insulin, and carotid arterial wall thickness: the ARIC study. Atherosclerosis Risk in Communities Study. J. Clin. Epidemiol., 48, 927–940.
68 Van Leer, E. M., Seidell, J. C. & Kromhout, D. (1995) Dietary calcium, potassium, magnesium and blood pressure in the Netherlands. Int. J. Epidemiol., 24, 1117–1123.
69 Kozielec, P. et al. (2005) Assessment of serum ionized magnesium levels in healthy volunteers, in patients with coronary artery disease and/or hypertension and in hypertension alone. Magnes. Res., 18, 241–244.
70 Zhang, X. et al. (2016) Effects of magnesium supplementation on blood pressure: A meta-analysis of randomized double-blind placebo-controlled trials. Hypertens. (Dallas, Tex. 1979), 68, 324–333.
71 Rosanoff, A. & Plesset, M. R. (2013) Oral magnesium supplements decrease high blood pressure (SBP > 155 mmHg) in hypertensive subjects on anti-hypertensive medications: A targeted meta-analysis. Magnes. Res., 26, 93–99.
72 Dibaba, D. T. et al. (2017) The effect of magnesium supplementation on blood pressure in individuals with insulin resistance, prediabetes, or noncommunicable chronic diseases: a meta-analysis of randomized controlled trials. Am. J. Clin. Nutr., 106, 921–929.
73 Qu, X. et al. (2013) Magnesium and the risk of cardiovascular events: a meta-analysis of prospective cohort studies. PLoS One, 8, e57720.
74 Fang, X. et al. (2016) Dietary magnesium intake and the risk of cardiovascular disease, type 2 diabetes, and all-cause mortality: a dose-response meta-analysis of prospective cohort studies. BMC Med., 14, 210.
75 Rodriguez-Ortiz, M. E. et al. (2019) Serum magnesium is associated with carotid atherosclerosis in patients with high cardiovascular risk (CORDIOPREV Study). Sci. Rep., 9, 8013.
76 Atabek, M. E., Kurtoglu, S., Pirgon, O. & Baykara, M. (2006) Serum magnesium concentrations in type 1 diabetic patients: relation to early atherosclerosis. Diabetes Res. Clin. Pract., 72, 42–47.
77 Posadas-Sanchez, R. et al. (2016) Serum magnesium is inversely associated with coronary artery calcification in the Genetics of Atherosclerotic Disease (GEA) study. Nutr. J., 15, 22.
78 Lee, S. Y., Hyun, Y. Y., Lee, K. B. & Kim, H. (2015) Low serum magnesium is associated with coronary artery calcification in a Korean population at low risk for cardiovascular disease. Nutr. Metab. Cardiovasc. Dis., 25, 1056–1061.
79 Hruby, A. et al. (2014) Magnesium intake is inversely associated with coronary artery calcification: The Framingham Heart Study. JACC Cardiovasc. Imaging, 7, 59–69.
80 Steidl, L. & Ditmar, R. (1990) Soft tissue calcification treated with local and oral magnesium therapy. Magnes. Res., 3, 113–119.
81 Cunha, A. R. et al. (2017) Oral magnesium supplementation improves endothelial function and attenuates subclinical atherosclerosis in thiazide-treated hypertensive women. J. Hypertens., 35, 89–97.
82 Erem, S., Atfi, A. & Razzaque, M. S. (2019) Anabolic effects of vitamin D and magnesium in aging bone. J. Steroid Biochem. Mol. Biol., 193, 105,400.
83 Reddy, V. & Sivakumar, B. (1974) Magnesium-dependent vitamin-D-resistant rickets. Lancet, 303, 963–965.
84 Saito, N. et al. (2004) Bone mineral density, serum albumin and serum magnesium. J. Am. Coll. Nutr., 23, 701S-703S.
85 Tucker, K. L. et al. (1999) Potassium, magnesium, and fruit and vegetable intakes are associated with greater bone mineral density in elderly men and women. Am. J. Clin. Nutr., 69, 727–736.
86 Tranquilli, A. L., Lucino, E., Garzetti, G. G. & Romanini, C. (1994) Calcium, phosphorus and magnesium intakes correlate with bone mineral content in postmenopausal women. Gynecol. Endocrinol., 8, 55–58.
87 Veronese, N. et al. (2017) Dietary magnesium intake and fracture risk: Data from a large prospective study. Br. J. Nutr., 117, 1570–1576.
88 Wang, M. C. et al. (1999) Influence of pre-adolescent diet on quantitative ultrasound measurements of the calcaneus in young adult women. Osteoporos. Int., 9, 532–535.
89 Zheng, J. et al. (2014) Association between serum level of magnesium and postmenopausal osteoporosis: a meta-analysis. Biol. Trace Elem. Res., 159, 8–14.
90 Castiglioni, S., Cazzaniga, A., Albisetti, W. & Maier, J. A. M. (2013) Magnesium and osteoporosis: current state of knowledge and future research directions. Nutrients, 5, 3022–3033.
91 Farsinejad-Marj, M., Saneei, P. & Esmaillzadeh, A. (2016) Dietary magnesium intake, bone mineral density and risk of fracture: a systematic review and meta-analysis. Osteoporos. Int., 27, 1389–1399.
92 Dimai, H. P. et al. (1998) Daily oral magnesium supplementation suppresses bone turnover in young adult males. J. Clin. Endocrinol. Metab., 83, 2742–2748.
93 Aydin, H. et al. (2010) Short-term oral magnesium supplementation suppresses bone turnover in postmenopausal osteoporotic women. Biol. Trace Elem. Res., 133, 136–143.
94 Carpenter, T. O. et al. (2006) A randomized controlled study of effects of dietary magnesium oxide supplementation on bone mineral content in healthy girls. J. Clin. Endocrinol. Metab., 91, 4866–4872.
95 Stendig-Lindberg, G., Tepper, R. & Leichter, I. (1993) Trabecular bone density in a two year controlled trial of peroral magnesium in osteoporosis. Magnes. Res., 6, 155–163.
96 Mao, S., Wu, L. & Shi, W. (2018) Association between trace elements levels and asthma susceptibility. Respir. Med., 145, 110–119.
97 Daliparty, V. M., Manu, M. K. & Mohapatra, A. K. (2018) Serum magnesium levels and its correlation with level of control in patients with asthma: A hospital-based, cross-sectional, prospective study. Lung India, 35, 407–410.
98 Kilic, H. et al. (2018) The relationship between hypomagnesemia and pulmonary function tests in patients with chronic asthma. Med. Princ. Pract., 27, 139–144.
99 Shaikh, M. N., Malapati, B. R., Gokani, R., Patel, B. & Chatriwala, M. (2016) Serum magnesium and vitamin D levels as indicators of asthma severity. Pulm. Med., 2016, 1,643,717.
100 Chitamanni, P., Chandrasekaran, V. & Rajendiran, S. (2018) Serum total magnesium level and its correlation with symptom control in children with mild persistent asthma. Indian J. Pediatr., 85, 420–425.
101 Sein, H. H. et al. (2014) Relationship between intracellular magnesium level, lung function, and level of asthma control in children with chronic bronchial asthma. Malays. J. Med. Sci., 21, 30–36.
102 Su, Z., Li, R. & Gai, Z. (2018) Intravenous and nebulized magnesium sulfate for treating acute asthma in children: A systematic review and meta-analysis. Pediatr. Emerg. Care, 34, 390–395.
103 Irazuzta, J. E. & Chiriboga, N. (2017) Magnesium sulfate infusion for acute asthma in the emergency department. J. Pediatr. (Rio. J)., 93 Suppl 1, 19–25.
104 Kew, K. M., Kirtchuk, L., Michell, C. I. & Griffiths, B. (2014) Intravenous magnesium sulfate for treating adults with acute asthma in the emergency department. Cochrane Database Syst. Rev., 2014, CD010909.
105 Javor, E. & Grle, S. P. (2019) Limitations of the results from randomized clinical trials involving intravenous and nebulised magnesium sulphate in adults with severe acute asthma. Pulm. Pharmacol. Ther., 55, 31–37.
106 Normansell, R. et al. (2018) Inhaled magnesium sulfate in the treatment of acute asthma in children. Paediatr. Respir. Rev., 26, 31–33.
107 Knightly, R. et al. (2017) Inhaled magnesium sulfate in the treatment of acute asthma. Cochrane Database Syst. Rev., 11, CD003898.
108 Abuabat, F. et al. (2019) The role of oral magnesium supplements for the management of stable bronchial asthma: a systematic review and meta-analysis. NPJ Prim. Care Respir. Med., 29, 4.
109 Petrov, V. I., Shishimorov, I. N., Perminov, A. A. & Nefedov, I. V. (2014) [Influence of magnesium deficiency correction on the effectiveness of bronchial asthma pharmacotherapy in children]. Eksp. Klin. Farmakol., 77, 23−27.
110 Kazaks, A. G., Uriu-Adams, J. Y., Albertson, T. E., Shenoy, S. F. & Stern, J. S. (2010) Effect of oral magnesium supplementation on measures of airway resistance and subjective assessment of asthma control and quality of life in men and women with mild to moderate asthma: a randomized placebo controlled trial. J. Asthma, 47, 83–92.
111 Bede, O., Suranyi, A., Pinter, K., Szlavik, M. & Gyurkovits, K. (2003) Urinary magnesium excretion in asthmatic children receiving magnesium supplementation: a randomized, placebo-controlled, double-blind study. Magnes. Res., 16, 262–270.
112 Guo, B.-L. et al. (2015) Effects of systemic magnesium on post-operative analgesia: Is the current evidence strong enough? Pain Physician, 18, 405–418.
113 Borazan, H., Kececioglu, A., Okesli, S. & Otelcioglu, S. (2012) Oral magnesium lozenge reduces postoperative sore throat: A randomized, prospective, placebo-controlled study. Anesthesiology, 117, 512–518.
114 Yousef, A. A. & Al-deeb, A. E. (2013) A double-blinded randomised controlled study of the value of sequential intravenous and oral magnesium therapy in patients with chronic low back pain with a neuropathic component. Anaesthesia, 68, 260–266.
115 Pickering, G. et al. (2011) Oral magnesium treatment in patients with neuropathic pain: A randomized clinical trial. Magnes. Res., 24, 28–35.
116 Proctor, M. L. & Murphy, P. A. (2001) Herbal and dietary therapies for primary and secondary dysmenorrhoea. Cochrane Database Syst. Rev., CD002124, doi:10.1002/14651858.CD002124.
117 Pirnia, B. et al. (2020) Effect of magnesium sulfate added to tincture of opium and buprenorphine on pain and quality of life in women with dysmenorrhea: A prospective, randomized, double-blind, placebo-controlled trial. Addict. Heal., 12, 259–268.
118 Dolati, S., Rikhtegar, R., Mehdizadeh, A. & Yousefi, M. (2019) The role of magnesium in pathophysiology and migraine treatment. Biol. Trace Elem. Res., doi:10.1007/s12011-019-01931-z.
119 Chiu, H.-Y., Yeh, T.-H., Huang, Y.-C. & Chen, P.-Y. (2016) Effects of intravenous and oral magnesium on reducing migraine: A meta-analysis of randomized controlled trials. Pain Physician, 19, E97−E112.
120 Karimi, N., Razian, A. & Heidari, M. (2019) The efficacy of magnesium oxide and sodium valproate in prevention of migraine headache: a randomized, controlled, double-blind, crossover study. Acta Neurol. Belg., doi:10.1007/s13760-019-01101-x.
121 Kovacevic, G. et al. (2017) A 6-month follow-up of disability, quality of life, and depressive and anxiety symptoms in pediatric migraine with magnesium prophylaxis. Magnes. Res., 30, 133–141.
122 Cohen, J. S. (2002) High-dose oral magnesium treatment of chronic, intractable erythromelalgia. Ann. Pharmacother., 36, 255–260.
123 (2017) Erythromelalgia. https://www.nhs.uk/conditions/erythromelalgia/
124 Andretta, A. et al. (2019) Relation between magnesium and calcium and parameters of pain, quality of life and depression in women with fibromyalgia. Adv. Rheumatol. (London, England), 59, 55.
125 Sakarya, S. T., Akyol, Y., Bedir, A. & Canturk, F. (2011) The relationship between serum antioxidant vitamins, magnesium levels, and clinical parameters in patients with primary fibromyalgia syndrome. Clin. Rheumatol., 30, 1039–1043.
126 Bagis, S. et al. (2013) Is magnesium citrate treatment effective on pain, clinical parameters and functional status in patients with fibromyalgia? Rheumatol. Int., 33, 167–172.
127 Russell, I. J., Michalek, J. E., Flechas, J. D. & Abraham, G. E. (1995) Treatment of fibromyalgia syndrome with Super Malic: a randomized, double blind, placebo controlled, crossover pilot study. J. Rheumatol., 22, 953−958.
128 Cox, I. M., Campbell, M. J. & Dowson, D. (1991) Red blood cell magnesium and chronic fatigue syndrome. Lancet (London, England), 337, 757–760.
129 Bjorklund, G., Dadar, M., Pen, J. J., Chirumbolo, S. & Aaseth, J. (2019) Chronic fatigue syndrome (CFS): Suggestions for a nutritional treatment in the therapeutic approach. Biomed. Pharmacother., 109, 1000–1007.
130 Makrides, M., Crosby, D. D., Bain, E. & Crowther, C. A. (2014) Magnesium supplementation in pregnancy. Cochrane Database Syst. Rev., CD000937, doi:10.1002/14651858.CD000937.pub2.
131 Ephraim, R. K. D. et al. (2014) Serum calcium and magnesium levels in women presenting with pre-eclampsia and pregnancy-induced hypertension: a case-control study in the Cape Coast metropolis, Ghana. BMC Pregnancy Childbirth, 14, 390.
132 He, L., Lang, L., Li, Y., Liu, Q. & Yao, Y. (2016) Comparison of serum zinc, calcium, and magnesium concentrations in women with pregnancy-induced hypertension and healthy pregnant women: A meta-analysis. Hypertens. Pregnancy, 35, 202–209.
133 Cabarkapa, V. et al. (2018) Serum magnesium level in the first trimester of pregnancy as a predictor of pre-eclampsia − a pilot study. Hypertens. Pregnancy, 37, 144–153.
134 Jafrin, W. et al. (2014) An evaluation of serum magnesium status in pre-eclampsia compared to the normal pregnancy. Mymensingh Med. J., 23, 649–653.
135 Zarean, E. & Tarjan, A. (2017) Effect of magnesium supplement on pregnancy outcomes: A randomized control trial. Adv. Biomed. Res., 6, 109.
136 You, H. J., Cho, S.-E., Kang, S.-G., Cho, S.-J. & Na, K.-S. (2018) Decreased serum magnesium levels in depression: a systematic review and meta-analysis. Nord. J. Psychiatry, 72, 534–541.
137 Li, B., Lv, J., Wang, W. & Zhang, D. (2017) Dietary magnesium and calcium intake and risk of depression in the general population: A meta-analysis. Aust. N. Z. J. Psychiatry, 51, 219–229.
138 Tarleton, E. K., Kennedy, A. G., Rose, G. L., Crocker, A. & Littenberg, B. (2019) The association between serum magnesium levels and depression in an adult primary care population. Nutrients, 11, 1475.
139 Sun, C., Wang, R., Li, Z. & Zhang, D. (2019) Dietary magnesium intake and risk of depression. J. Affect. Disord., 246, 627–632.
140 Martinez-Gonzalez, M. A. & Sanchez-Villegas, A. (2016) Magnesium intake and depression: the SUN cohort. Magnes. Res., 29, 102–111.
141 Ryszewska-Pokrasniewicz, B. et al. (2018) Effects of magnesium supplementation on unipolar depression: A placebo-controlled study and review of the importance of dosing and magnesium status in the therapeutic response. Nutrients, 10, 1014.
142 Fard, F. E. et al. (2017) Effects of zinc and magnesium supplements on postpartum depression and anxiety: A randomized controlled clinical trial. Women Health, 57, 1115–1128.
143 Rajizadeh, A., Mozaffari-Khosravi, H., Yassini-Ardakani, M. & Dehghani, A. (2017) Effect of magnesium supplementation on depression status in depressed patients with magnesium deficiency: A randomized, double-blind, placebo-controlled trial. Nutrition, 35, 56–60.
144 Boyle, N. B., Lawton, C. & Dye, L. (2017) The effects of magnesium supplementation on subjective anxiety and stress−A systematic review. Nutrients, 9, 429.
145 Moslehi, M., Arab, A., Shadnoush, M. & Hajianfar, H. (2019) The association between serum magnesium and premenstrual syndrome: A systematic review and meta-analysis of observational studies. Biol. Trace Elem. Res., 192, 145–152.
146 Fathizadeh, N., Ebrahimi, E., Valiani, M., Tavakoli, N. & Yar, M. H. (2010) Evaluating the effect of magnesium and magnesium plus vitamin B6 supplement on the severity of premenstrual syndrome. Iran. J. Nurs. Midwifery Res., 15, 401–405.
147 Ebrahimi, E., Khayati Motlagh, S., Nemati, S. & Tavakoli, Z. (2012) Effects of magnesium and vitamin b6 on the severity of premenstrual syndrome symptoms. J. Caring Sci., 1, 183–189.
148 Quaranta, S., Buscaglia, M. A., Meroni, M. G., Colombo, E. & Cella, S. (2007) Pilot study of the efficacy and safety of a modified-release magnesium 250 mg tablet (Sincromag) for the treatment of premenstrual syndrome. Clin. Drug Investig., 27, 51–58.
149 & Mitmesser, S. H. (2019) Micronutrient inadequacy in short sleep: Analysis of the NHANES 2005−2016. Nutrients, 11, 2335.
150 van Ooijen, G. & O’Neill, J. S. (2016) Intracellular magnesium and the rhythms of life. Cell Cycle (Georgetown, Tex.), 15, 2997–2998.
151 Dralle, D. & Bodeker, R. H. (1980) Serum magnesium level and sleep behavior of newborn infants. Eur. J. Pediatr., 134, 239–243.
152 Abbasi, B. et al. (2012) The effect of magnesium supplementation on primary insomnia in elderly: A double-blind placebo-controlled clinical trial. J. Res. Med. Sci., 17, 1161–1169.
153 Rondanelli, M. et al. (2011) The effect of melatonin, magnesium, and zinc on primary insomnia in long-term care facility residents in Italy: A double-blind, placebo-controlled clinical trial. J. Am. Geriatr. Soc., 59, 82–90.
154 Szentkiralyi, A. et al. (2019) Prevalence and associated risk factors of periodic limb movement in sleep in two German population-based studies. Sleep, 42, zsy237.
155 Marshall, N. S. et al. (2019) Magnesium supplementation for the treatment of restless legs syndrome and periodic limb movement disorder: A systematic review. Sleep Med. Rev., 48, 101,218.
156 Hornyak, M., Voderholzer, U., Hohagen, F., Berger, M. & Riemann, D. (1998) Magnesium therapy for periodic leg movements-related insomnia and restless legs syndrome: An open pilot study. Sleep, 21, 501–505.
157 Mendelski, B. (2005) Wirksamkeit von Magnesium in der Behandlung des idiopathischen Restless Legs Syndroms: ergebnisse einer placebo-kontrollierten, randomisierten Doppelblindstudie [Efficacy of magnesium in the treatment of idiopathic restless legs syndrome: results of a placebo-controlled, randomized double blind study]. Albert-Ludwigs-Universität Freiburg.
158 Sebo, P., Cerutti, B. & Haller, D. M. (2014) Effect of magnesium therapy on nocturnal leg cramps: A systematic review of randomized controlled trials with meta-analysis using simulations. Fam. Pract., 31, 7–19.
159 Garrison, S. R., Allan, G. M., Sekhon, R. K., Musini, V. M. & Khan, K. M. (2012) Magnesium for skeletal muscle cramps. Cochrane Database Syst. Rev., CD009402, doi:10.1002/14651858.CD009402.pub2.
160 Roguin Maor, N. et al. (2017) Effect of magnesium oxide supplementation on nocturnal leg cramps: A randomized clinical trial. JAMA Intern. Med., 177, 617–623.
161 Izzo, A. A., Gaginella, T. S. & Capasso, F. (1996) The osmotic and intrinsic mechanisms of the pharmacological laxative action of oral high doses of magnesium sulphate. Importance of the release of digestive polypeptides and nitric oxide. Magnes. Res., 9, 133–138.
162 Murakami, K. et al. (2007) Association between dietary fiber, water and magnesium intake and functional constipation among young Japanese women. Eur. J. Clin. Nutr., 61, 616–622.
163 Dupont, C., Campagne, A. & Constant, F. (2014) Efficacy and safety of a magnesium sulfate-rich natural mineral water for patients with functional constipation. Clin. Gastroenterol. Hepatol., 12, 1280–1287.
164 Bothe, G., Coh, A. & Auinger, A. (2017) Efficacy and safety of a natural mineral water rich in magnesium and sulphate for bowel function: a double-blind, randomized, placebo-controlled study. Eur. J. Nutr., 56, 491–499.
165 Naumann, J., Sadaghiani, C., Alt, F. & Huber, R. (2016) Effects of sulfate-rich mineral water on functional constipation: A double-blind, randomized, placebo-controlled study. Forsch. Komplementmed., 23, 356–363.
166 Dupont, C. et al. (2019) Time to treatment response of a magnesium- and sulphate-rich natural mineral water in functional constipation. Nutrition, 65, 167–172.
167 Mori, S. et al. (2019) A randomized double-blind placebo-controlled trial on the effect of magnesium oxide in patients with chronic constipation. J. Neurogastroenterol. Motil., 25, 563–575.
168 Kinnunen, O. & Salokannel, J. (1987) Constipation in elderly long-stay patients: Its treatment by magnesium hydroxide and bulk-laxative. Ann. Clin. Res., 19, 321–323.
169 Ni, Y. H., Lin, C. C., Chang, S. H. & Yeung, C. Y. (2001) Use of cisapride with magnesium oxide in chronic pediatric constipation. Acta Paediatr. Taiwan, 42, 345–349.
170 Gomes, P. B., Duarte, M. A. & Melo, M. do C. B. de. (2011) Comparison of the effectiveness of polyethylene glycol 4000 without electrolytes and magnesium hydroxide in the treatment of chronic functional constipation in children. J. Pediatr. (Rio. J.), 87, 24–28
171 Benninga, M. A. & Vandenplas, Y. (2019) The magnesium-rich formula for functional constipation in infants: A randomized comparator-controlled study. Pediatr. Gastroenterol. Hepatol. Nutr., 22, 270–281.
172 Xinias, I. et al. (2018) A synbiotic infant formula with high magnesium content improves constipation and quality of life. Pediatr. Gastroenterol. Hepatol. Nutr., 21, 28–33.
173 Mori, H. et al. (2019) Clinical features of hypermagnesemia in patients with functional constipation taking daily magnesium oxide. J. Clin. Biochem. Nutr., 65, 76–81.
174 Wakai, E., Ikemura, K., Sugimoto, H., Iwamoto, T. & Okuda, M. (2019) Risk factors for the development of hypermagnesemia in patients prescribed magnesium oxide: A retrospective cohort study. J. Pharm. Heal. Care Sci., 5, 4.
175 Maton, P. N. & Burton, M. E. (1999) Antacids revisited: A review of their clinical pharmacology and recommended therapeutic use. Drugs, 57, 855–870.
176 NICE (2019) BNF. BNF, https://bnf.nice.org.uk/treatment-summary/antacids.html
177 NHS (2019) Antacids. https://www.nhs.uk/conditions/antacids/.
178 Thomas, D. (2007) The mineral depletion of foods available to us as a nation (1940−2002)−a review of the 6th Edition of McCance and Widdowson. Nutr. Health, 19, 21–55.
179 Mayer, A.-M. (1997) Historical changes in the mineral content of fruits and vegetables. Br. Food J., 99, 207–211.
180 Higdon, J. & Volpe, S. L. (2019) Magnesium, https://lpi.oregonstate.edu/mic/minerals/magnesium.
181 Harding, M. (2014) Magnesium disorders. patient.info https://patient.info/doctor/magnesium-disorders#.
182 Lewis, J. L. (2018) Hypermagnesaemia. MSD Manual, https://www.msdmanuals.com/en-gb/professional/endocrine-and-metabolic-disorders/electrolyte-disorders/hypermagnesemia.
183 EFSA (2006) Tolerable upper intake levels for vitamins and minerals. http://www.efsa.europa.eu/sites/default/files/efsa_rep/blobserver_assets/ndatolerableuil.pdf.
184 Kashihara, Y. et al. (2019) Effects of magnesium oxide on pharmacokinetics of L-dopa/carbidopa and assessment of pharmacodynamic changes by a model-based simulation. Eur. J. Clin. Pharmacol., 75, 351–361.
185 Brown, D. D. & Juhl, R. P. (1976) Decreased bioavailability of digoxin due to antacids and kaolin-pectin. N. Engl. J. Med., 295, 1034–1037.
186 Kivisto, K. T. & Neuvonen, P. J. (1991) Enhancement of absorption and effect of glipizide by magnesium hydroxide. Clin. Pharmacol. Ther., 49, 39–43.
187 Neuvonen, P. J. & Kivisto, K. T. (1991) The effects of magnesium hydroxide on the absorption and efficacy of two glibenclamide preparations. Br. J. Clin. Pharmacol., 32, 215–220.