By Chloe Steele
Abstract
Oral Lactobacillus rhamnosus GG (LGG) supplementation is generally recognised as a safe form of supplementation, which acts as an immunomodulator, an antimicrobial, and aids cell growth and proliferation. The aim of this review was to determine diseases where oral LGG supplementation has been indicated; and assess safety, colonisation, mechanisms of action and efficacy, and provide therapeutic recommendations. LGG following supplementation can successfully colonise the gut and other areas of the body owing to the expression of unique morphological features known as pili. Twenty-two disease areas were identified where LGG supplementation has been used, to determine effects. However, small study sizes, the use of multispecies probiotics and adjuvant therapies all meant that strong evidence for the use of LGG was lacking in several disease areas. Despite this, LGG was shown to be of benefit in the reduction of risk of developing attention-deficit hyperactivity disorder and gestational diabetes mellitus, in the prevention of allergies and dental caries, for improving immune reactions following vaccines, and for the management of diarrhoea associated with cancer treatments and antibiotic use.
Cite as: Steele, C. (2022) Lactobacillus rhamnosus GG: a review of clinical use and efficacy. Nutr. Med. J., 1 (3), 70-116.
Affiliation: C. Steele is with the Nutritional Medicine Institute, London, England, and the Centre for Nutrition Education & Lifestyle Management (CNELM), Wokingham, England.
Corresponding author: Chloe Steele (email chloe-steele@ac.cnelm.co.uk).
Article history: Received 17 December 2021; Peer-reviewed and received in revised form 10 August 2022; Accepted 12 August 2022. Available online 30 September 2022.
Published by: The Nutritional Medicine Institute
Open Access: This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs licence (http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reproduction and distribution of the work, in any medium, provided the original work is not altered or transformed in any way, and that the work is properly cited. For commercial use please contact support@nmi.health
Introduction
Probiotics are defined as live microbes, which when administered in adequate amounts confer benefits to the host.1 For this to occur, probiotics need to be safe, alive, of human origin and capable of surviving the pH of the gut.2 Several different bacteria are used as probiotics, but species from the genera Lactobacillus and Bifidobacterium have been well researched and are believed to provide many health benefits.3,4,5,6
Amongst the most well-researched strains of probiotics is Lactobacillus rhamnosus GG (LGG). Its health benefits are thought to derive from its superior ability to colonise the gastrointestinal (GI) tract, outcompeting and producing antimicrobials to prevent pathogenic bacterial colonisation.7 LGG may also promote GI barrier protection and healing through cell growth and proliferation,8,9 and act as an immune effector both locally and systemically.10 Based on the mechanisms of action, clinical trials in humans have been extensive, showing benefits in several disease areas.
This review paper aims to determine the disease areas where the use of LGG as an oral probiotic has been indicated, and review the clinical data on efficacy and safety with a view to making therapy recommendations. Data on the mechanistic actions of LGG will also be briefly reviewed. Randomised-controlled trials (RCTs) will dominate this review, as the beneficial effects of probiotics seem to be strain specific;11 thus, pooling data in large meta-analyses and systematic reviews with different strains may result in misleading conclusions. Where LGG alone has been examined, these will be included.
Colonisation and adhesion
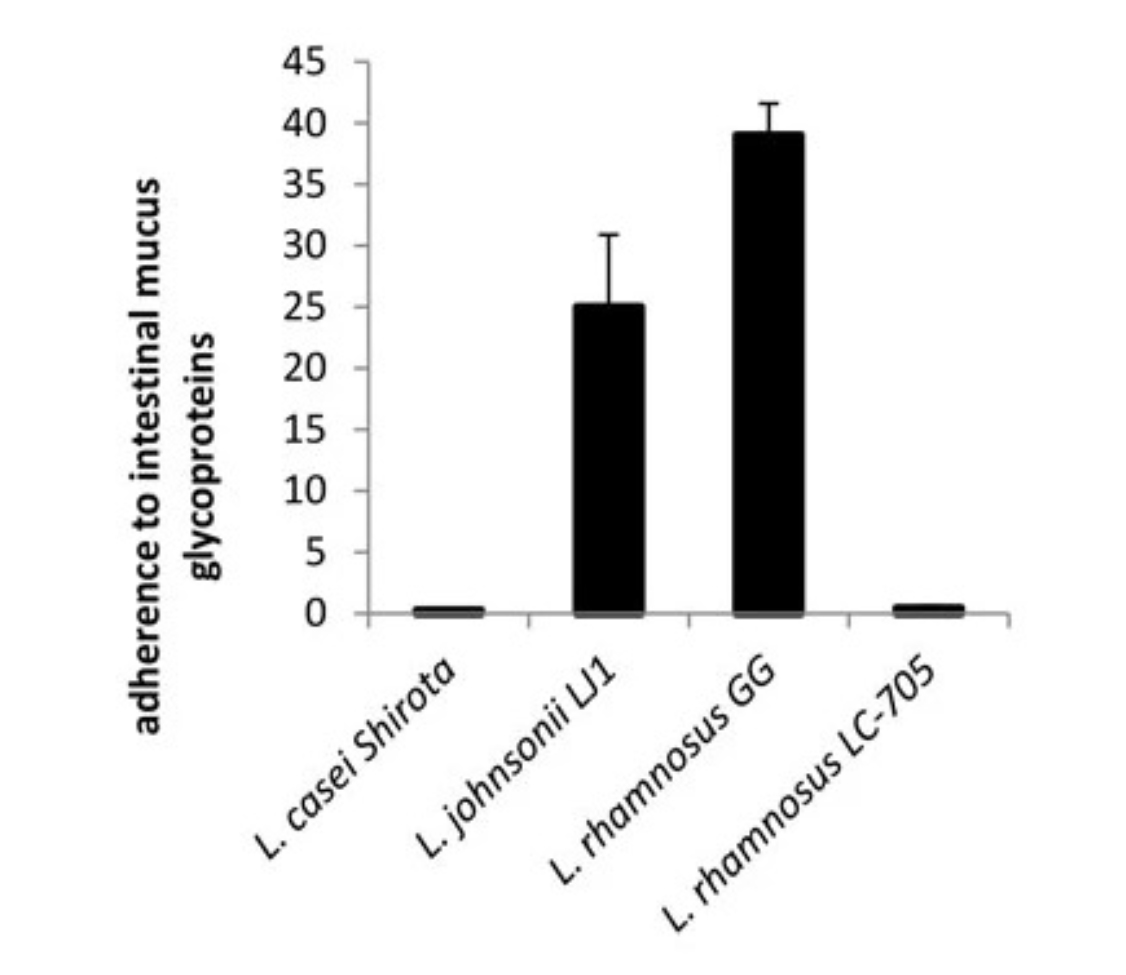
The success of probiotic supplementation relies upon the ability of the microbiota to colonise areas of the body, such as the GI tract. In comparison to other Lactobacillus strains, LGG has high adherence to human intestinal mucus glycoproteins7 and, in adults, supplementation has indicated that it may survive for at least 1 week in the GI tract12 (Figure 1). Amongst newborns and infants, a reduced intrinsic GI microbiome may ensure that LGG colonises the GI tract more readily, and it has been detected in faeces up to 2 weeks after administration, without affecting the establishment of a normal GI microbiome.13
Image 1: LGG has superior mucus adherence
Figure 1: Lactobacillus rhamnosus GG (LGG) has superior adherence to mucus glycoproteins when compared with other probiotic strains, including a closely related strain of L. rhamnosus (LC705). In this study, radioactively labelled bacteria were allowed to adhere to isolated human intestinal mucus. The adhesion ratio (%) was determined by comparing radioactivity of bacteria added to the radioactivity
of bound bacteria after washing.
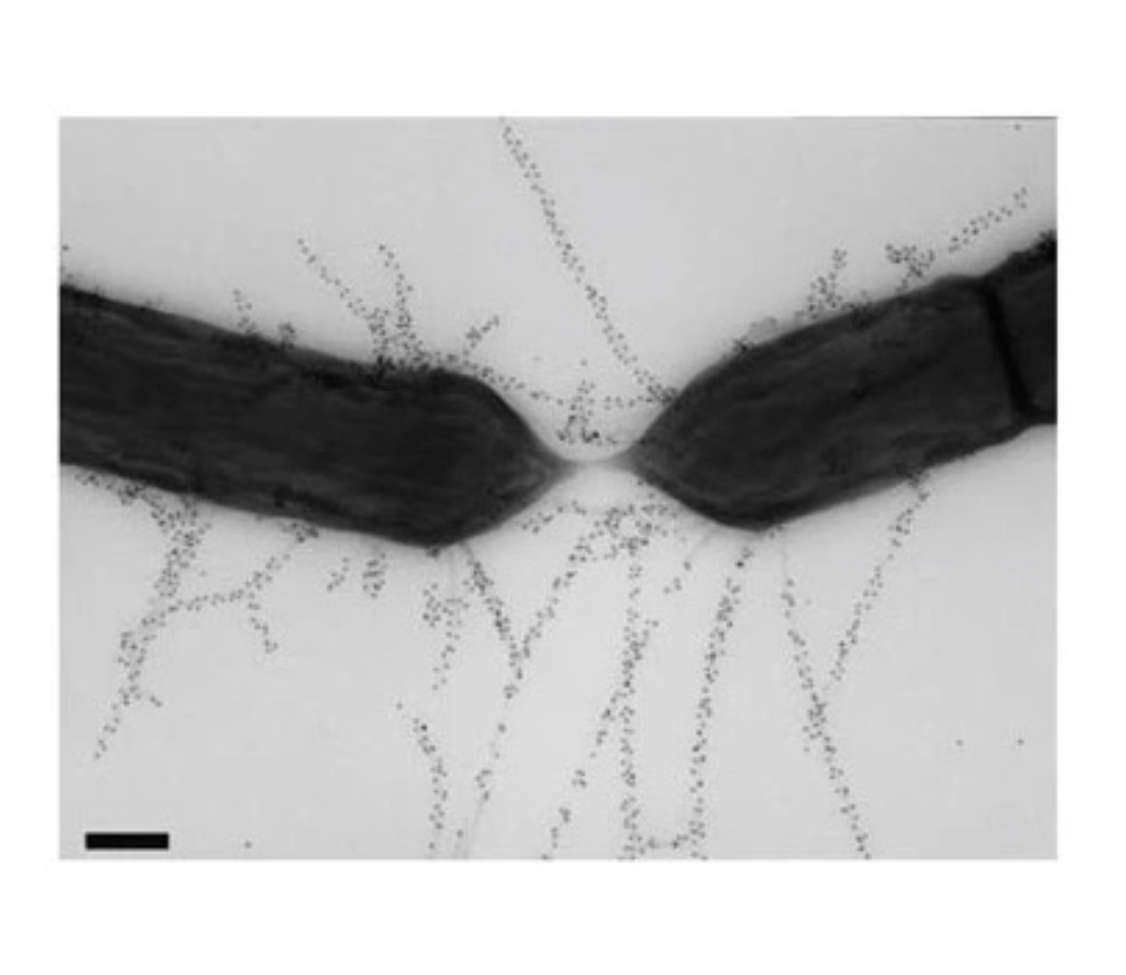
Successful colonisation of LGG, compared with other Lactobacillus species, may be down to certain morphological features. Comparative genomic analysis with Lactobacillus rhamnosus LC705 has revealed the presence of a DNA sequence, known as the spaCBA gene, resulting in pili-like appendages that run along the entirety of the LGG microbe, the inhibition of which lowers adhesion to the GI tract14 (Figure 2). Differing conditions have been shown to promote or suppress the expression of the pili phenotype in other microbiota strains;15 however, when exposed to different conditions such as low pH, the pili of LGG are still expressed.16 Interestingly, whilst LGG still expresses pili under different conditions, when present in the oral and vaginal cavities the pili are absent,16 which may have implications in diseases associated within these areas, such as urinary tract infections (UTIs) and dental caries.
Image 2: Lactobacillus rhamnosus GG-specific pili
Figure 2: Lactobacillus rhamnosus GG (LGG)-specific pili, not present on other Lactobacillus spp., are involved in the mechanisms of adhesion to the intestinal mucosa. In addition, the pili facilitate a close interaction between the host and the bacteria or bacteria with each other. In this image, transmission electron microscopy reveals pili on LGG cells.
Certain probiotic strains have been shown to adhere to the GI tract, preventing mucolytic bacteria from digesting the protective layer of mucus, resulting in decreased vulnerability to intestinal permeability.17 Although studies have shown limited effects of LGG on intestinal permeability in patients with chronic liver conditions,18 investigations into efficacy in healthy patients is warranted.
General effects
Immunomodulation
The role of LGG in immunomodulation is controversial, with proteins isolated from it and its physico-chemical properties contributing to both inflammatory and anti-inflammatory actions. The pili on LGG have been implicated to have a role in immunomodulation. An in vitro study on LGG bred without the spaCBA gene, which encodes for the growth of the external pili, reported increased expression of the inflammatory cytokine interleukin (IL)-8, which was decreased with the wild-type strain.19 Proteins secreted from LGG may also have an anti-inflammatory role in the immune response, and isolation of a novel soluble protein, HM0539, from LGG has been shown in colon tissue to suppress the TLR4/MyD88/NFкB inflammatory pathway.20 However, overexpression of toll-like receptor (TLR)4 or myeloid differentiation primary response 88 (MyD88) did reverse this effect. The TLR4 signalling pathway may be responsible for upregulated inflammation in chronic and acute inflammatory disorders,21,22 such as inflammatory bowel disease (IBD) and atherosclerosis,23,24 indicating that LGG supplementation in highly inflammatory states may have a limited effect.
In contrast to its anti-inflammatory effects, effector substances such as lipoteichoic acid (LTA), found in the cell walls of certain gram-positive bacteria including LGG, may also be involved in the modulation of the immune response, with it displaying pro-inflammatory properties. Within the body, reporter cell lines are designed to monitor intracellular cell signalling pathways. LTA produced by a wild-type LGG strain has been shown to activate the inflammatory NF-кB signalling pathway in reporter cells, a pathway that is significantly reduced when the LTA gene is removed.10 Removal of the LTA gene resulted in a reduced capacity to activate TLR2/6-dependent NFкB signalling in reporter cells and reduced induction of IL-8 mRNA in CACO-2 cells from the human colon, acting both locally and systemically. However, the implications of this during certain disease states and the exact role of LGG in the inflammatory and anti-inflammatory pathways still needs to be elucidated.
Supplementation of LGG in individuals with inflammatory GI diseases has shown mixed results, and is discussed later in the review.25,26,27
Cell growth and proliferation
Proteins produced by LGG, known as Msp1 and Msp2, have been implicated in cell homeostasis through regulation of the protein kinase B (Akt) signalling pathway and inhibition of MAP kinases.8,9 In vitro studies in animal and human colon tissue have shown that Msp1 and Msp2 promoted cell growth and attenuated GI permeability in hydrogen peroxide-damaged intestinal epithelium.8 Msp2 has also been shown in intestinal epithelial cells, in vivo and in vitro, to inhibit cytokine-induced apoptosis,28 indicating a role for LGG in the protection and recovery from intestinal permeability and injury.
Antimicrobial
In vitro, LGG has been shown to inhibit the growth and adherence of several pathogenic bacteria belonging to the Salmonella, Shigella, Escherichia and Streptococcus species.7,29,30,31,32 In rabbit models, LGG has been shown to inhibit translocation of Escherichia coli in a dose-dependent manner.33 In clinical trials, a decrease in the number of children colonised with vancomycin-resistant enterococci has been reported following LGG consumption for 21 days, with increased GI Lactobacillus counts observed in their stead.34
LGG DNA contains encodes for bacteriocins, which act like antibiotics, preventing the growth of closely related bacterial strains; however, the product of these genes has not been expressed under experimental conditions. Further experiments have reported that the antimicrobial action of LGG may be due to the production of microcin-type substances, which are small bacteriocins, mediated in part by lactic acid.32,35,36
LGG may also communicate with other gut microbiota via a process known as quorum sensing (QS), resulting in cooperation for nutrients and cellular adhesion against pathogenic bacteria.37 The pili-like protrusions responsible for colonisation may ensure superior competitive inhibition by LGG during QS. However, further studies are required to determine the role of QS when the GI tract is faced with pathogenic bacteria.
Clinical uses
Cancer
It has been hypothesised that gut dysbiosis may promote colorectal cancer through the colonisation of pathogenic bacteria, which drives its development.38 Furthermore, chemotherapy treatment may alter the composition of the gut microbiota,39 indicating areas where probiotics may be of benefit. The use of LGG in a multispecies probiotic in combination with a prebiotic has been shown to alter several colorectal cancer biomarkers after 12 weeks.40 In this trial, Bifidobacterium (P = 0.008), Lactobacillus (P = 0.021) and interferon-gamma (IFN-γ) were all increased, with Clostridium perfringens (P = 0.022) and DNA damage decreased amongst 37 patients with colon cancer and 43 polypectomised patients. However, administration of the synbiotic also prevented a rise in IL-2 inflammatory cytokines. In contrast to IL-2 suppression, a second RCT on a multispecies synbiotic containing LGG, Bifidobacterium lactis and inulin reported increased IL-2 and IFN-γ in 34 patients with colon cancer who had undergone curative resection or polypectomy.41 IFN-γ and IL-2 were both increased at 12 weeks compared with placebo (P ≤ 0.05 both), but no other effects on immune factors were observed. Overall, it is difficult to conclude any specific effects of LGG from these trials as, when in combination, effects may be due to other species.
Failure of cancer treatments often occurs when severe side-effects result in a reduction or cessation of treatment.42 Side-effects, such as diarrhoea, can occur in as many as 30−87% of patients, with severe and potentially life-threatening (grade 3−4) episodes occurring in 20−40% of patients.43 Probiotics have been shown to be a safe and effective way to prevent chemotherapy-induced diarrhoea39 and, as monotherapy, one of the most important effects of LGG may be for its use to reduce the frequency and severity of severe diarrhoea and GI symptoms during chemotherapy treatment. In one RCT of 150 patients with colorectal cancer given LGG twice daily [1−2 × 1010 colony-forming units (CFU)] for 24 weeks during 5-fluorouracil chemotherapy, patients had less grade 3 or 4 diarrhoea and fewer hospitalisations due to bowel toxicity compared with a fibre supplement (22% versus 37%, P = 0.027; and 8% versus 22%, P = 0.021, respectively), resulting in decreased chemotherapy dose adjustments (21% versus 47%, P = 0.0008).44
Perioperative administration of a multispecies probiotic containing LGG plus fructo-oligosaccharide has also been associated with reduced infection rate in postoperative patients with colorectal cancer. One trial in 91 patients undergoing surgery reported decreased infections at the incision site (2% versus 21.4%, P = 0.002), reduced intra-abdominal abscess (P ≤ 0.001) and reduced incidence of pneumonia (P ≤ 0.001), indicating a beneficial effect to complications associated with cancer treatment.3
Helicobacter pylori may be the strongest known risk factor for gastric cancer, and eradication may be an effective therapy in its prevention.45 However, undesirable side-effects of eradication may include diarrhoea, pain, nausea and bloating, resulting in treatment cessation.46 During H. pylori eradication, the supplementation of LGG (6 × 109 CFU twice daily) has been reported to increase eradication tolerability (P = 0.04) due to decreased side-effects, such as diarrhoea, nausea and taste disturbances [relative risk (RR) = 0.1, 95% confidence interval (CI): 0.1−0.9; RR = 0.3, 95% CI: 0.1−0.9; RR = 0.5, 95% CI: 0.2−0.9].47 Although eradication rate remained unaffected, the supplementation of LGG in individuals undergoing H. pylori eradication may contribute to preventing the development of gastric cancer through increased treatment tolerability.
Results have not been as positive in other cancers, with one study in 40 patients undergoing head and neck cancer surgery reporting no impact of a multispecies synbiotic on postoperative outcomes, intestinal function or GI symptoms,48 indicating that the beneficial effects of LGG in cancer may be localised and specific.
It is apparent that LGG monotherapy (6 × 109 CFU twice daily) has numerous benefits, including success in symptom management of H. pylori treatment and reducing the risk of developing gastric cancer. For individuals undergoing chemotherapy treatment for colon cancer, LGG (1−2 × 1010 CFU twice daily) may be more beneficial than fibre for the reduction of diarrhoea. In combination with other probiotics and prebiotics, LGG may reduce postoperative infections and improve immune function in patients with colon cancer, which is important during a time of reduced immune function.
Irritable bowel syndrome
Irritable bowel syndrome (IBS) is characterised by abdominal pain, flatulence and irregular bowel movements, and it is estimated that 10−20% of the worldwide adult population suffers from this syndrome.49 Treatments for IBS have limited success, and newer drugs such as 5-HT4 agonists come with cardiovascular risks,50 highlighting a need for treatments with limited side-effects.
In adults, it appears that several factors are involved in the pathophysiology of IBS, with mucosal large intestine low-grade inflammation and altered gut microbiota indicated.51 As stated earlier, in vitro studies have highlighted that LGG may have both pro-inflammatory and anti-inflammatory properties, making its role as a supplement in inflammatory diseases uncertain.
Differences in the gut microbiota between healthy subjects and sufferers of IBS have been highlighted.52,53 The administration of LGG may be able to promote colonisation and reinstate the composition of gut microbiota more associated with healthy individuals. Supplementation of a multispecies probiotic containing LGG (8−9 × 109 CFU) for 6 months in 42 patients with IBS resulted in a shift towards similar quantities of bacterial 16S rDNA to those of healthy controls; however, clinical outcomes were not reported in this study.54 Although there was a shift in composition towards that of more healthy individuals, a gut microbiota composition that is characteristic of, or favourable for, sufferers of IBS has yet to be isolated and may be highly individual.55 Regardless, the administration of probiotics containing LGG may be of benefit for symptom relief in individuals with IBS. In one double-blind RCT of 49 patients with IBS, the supplementation of a multispecies probiotic (5 × 109 CFU/day) containing LGG reported improvements to abdominal pain/discomfort and bloating after 4 weeks, which was attributed to alterations in the composition of the gut microbiota.27 Faecal analysis highlighted that LGG, B. lactis and Streptococcus thermophilus had all significantly increased in the supplemented group, although several other strains were also included in the probiotic.
IBS can be divided into subtypes,56 and improvements in symptoms may be highly dependent upon this, highlighting the importance of identifying the IBS type before commencing LGG supplementation. One 6-week unblinded RCT of 123 adults with IBS investigated the efficacy of LGG (6 × 109 CFU/day for 6 weeks) compared with a low-fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) diet on syndrome severity, assessed using the IBS symptom severity scoring system.26 The results showed that LGG was as efficacious as the low-FODMAP diet, especially in the diarrhoeal (IBS-D) and mixed IBS (IBS-A) subtypes, with no improvement in those with the IBS constipation subtype. Quality of life (QoL) was also improved in those with IBS-D. Studies like this highlight the need to perform trials on dual therapy of diet changes and probiotics to further advance possible management strategies for IBS.
Children with IBS may spontaneously recover;57 however, for those who have persistent symptoms, the use of LGG to effectively manage IBS may also be dependent on an accurate diagnosis. Improvements to the frequency and severity of abdominal pain when supplemented with LGG may be due to improvements in the gut barrier, and may be especially pronounced in children with IBS or functional pain.4 In one 16-week RCT of 141 children with IBS or functional pain, LGG supplementation (6 × 109 CFU/day) reduced the frequency and severity of abdominal pain (P ≤ 0.01 for both). Although it was concluded that this effect may not be unique to LGG, it is apparent that it may be of benefit. One systematic review of three RCTs with 290 children suffering from abdominal pain-related functional GI disorders concluded that a higher rate of children responded to treatment with LGG (6 × 109 CFU/day to 1010 CFU/day) compared with placebo if suffering from IBS, an effect that was not evident in children without IBS.58 Frequency of pain was only reduced in the IBS subgroup; however, pain intensity was reduced amongst the whole study population. This study did not perform a statistical test for publication bias, so this cannot be ruled out and conclusions should be made with caution.
A second systematic review of 11 RCTs examining various probiotic supplements in children with functional abdominal pain disorders (FAPD) highlighted that LGG reduces the frequency and severity of abdominal pain, but only in children with IBS.25 One study of 4 weeks, which included IBS sufferers alongside those with dyspepsia and functional abdominal pain, concluded that children supplemented with 3 × 109 CFU of LGG twice daily were more likely to have improved pain frequency but not pain severity,59 further supporting the need to identify IBS for LGG supplementation to be of benefit to symptoms.
In vitro studies have highlighted that LGG may have both pro-inflammatory and anti-inflammatory properties, and have a limited effect in inflammatory conditions. However, supplementation studies of the use of LGG to relieve symptoms of IBS in adults may be more positive, and dependent upon its use for the IBS-D and IBS-A subtypes, although this is based on data from studies using multispecies rather than LGG alone. Its use in children may be of benefit for the management of pain symptoms.
Diarrhoea
Diarrhoea is a leading cause of childhood mortality worldwide and is the second leading cause of childhood deaths.60 The use of probiotics and LGG for the prevention and treatment of diarrhoea has been extensively researched, with varying success. One large RCT of 943 children with moderate-to-severe gastroenteritis reported that administration of a short 5-day course of LGG 1 × 1010 CFU twice daily was no more effective than placebo at decreasing the duration of all-cause diarrhoea (median LGG 49.7 hours versus 50.9 hours, P = 0.26).61
Despite negative outcomes in all-cause diarrhoea, the understanding of the mechanisms through which LGG interacts with the host may indicate specific types of diarrhoea where supplementation may have greater success. Antibiotic-associated diarrhoea (AAD) may result from dysbiosis of the host’s gut bacteria.62 LGG acts through several mechanisms to potentially prevent dysbiosis or restore normal bacterial flora resulting from antibiotic administration, such as competitive exclusion of pathogens,63 modulation of the immune system,64 and through outcompeting less acid-tolerant bacteria as LGG produces lactic acid.65 The prevention of gut microbiota changes associated with antibiotic use has been shown in one RCT of 231 school-aged children.65 In this trial, children given 106 CFU/ml LGG in 400 ml of milk long term reported changes in several gut bacteria, with especially increased abundance of the Lactobacillus spp. (P < 0.0001).
LGG for the reduction of risk of AAD has been extensively researched, and it may be important for the prevention of this disease and to provide new treatment options when antibiotics are prescribed. One meta-analysis of 12 RCTs and 1499 participants reported that, compared with placebo or no treatment, LGG was associated with a reduced risk of AAD (22.4% to 12.3%, RR = 0.49, 95% CI: 0.29−0.83), resulting in a number needed to treat (NNT) of 9.66 LGG may also be one of the most effective probiotics for the prevention of AAD, and a meta-analysis of 32 RCTs has reported that LGG was superior to seven single or multispecies probiotics for the prevention of AAD (RR versus placebo = 0.30, 95% CI: 0.16−0.5).67 Dosages of at least 2 × 109 CFU were recommended.
Although evidence exists for the use of LGG with AAD, other causes of diarrhoea have reported mixed results. As previously discussed, in vitro studies have indicated the ability of LGG to prevent the adherence and viability of several gut pathogens.36,68 Amongst these, Clostridium spp. have been shown to be inhibited in vitro through the production of a bactericide that resembles microcin.32 However, in vivo studies in children have not been as positive. One meta-analysis of 20 RCTs concluded that the use of LGG reduced the risk of AAD from 23% to 9.6% (RR = 0.48, 95% CI: 0.26−0.89); however, it found no effect on the risk of Clostridium difficile-associated diarrhoea (RR = 0.95, 95% CI: 0.06−14.85).69 It should be noted that the results on C. difficile were based on only one RCT, and this warrants more research.
Studies on children with rotavirus-associated diarrhoea, which is the leading cause of vaccine-preventable diarrhoea,60 have been more positive. One meta-analysis of 19 RCTs concluded that, compared with control, the use of high-dose LGG (1010 CFU/day) in children with rotavirus-positive diarrhoea reduced the duration [mean difference (MD) −31.05 hours, 95% CI: −50.31, −11.80] and frequency of diarrhoea episodes (MD −1.08, 95% CI: −1.87, −0.28).70 This trial also looked at children with acute diarrhoea caused by a mixture of rotavirus, bacterial pathogens and norovirus and, in contrast to Schnadower et al. (2018),61 high-dose LGG reduced the duration of diarrhoea episodes (MD −15.83 hours, 95% CI: −20.68, −10.98), but only in those who had suffered from diarrhoea for less than 3 days at enrolment, indicating that earlier treatment at higher doses may have more success.
Dose-dependent effects of LGG supplementation on rotavirus-associated diarrhoea are also apparent. One open-label RCT in 23 children with rotavirus-associated diarrhoea showed no changes in faecal rotavirus concentrations when supplemented with low-dose LGG 2 × 108 CFU/day (36.1 × 105 particles/ml versus 73.5 × 105 particles/ml, P = 0.895), but at a high dose of 6 × 108 CFU/day concentrations were reduced (64.2 × 105 particles/ml versus 9.0 × 105 particles/ml, P = 0.012).71 Although rotavirus shedding and not symptoms were assessed in this trial, it is indicative of disease severity. LGG may also aid recovery from rotavirus infection, with both intestinal permeability and immunoglobulin antibodies to rotavirus improved following LGG supplementation.72
Large, short-term trials for the treatment of all-cause diarrhoea have shown little improvements with the administration of LGG. Understanding the type of diarrhoea may result in a more targeted and successful approach. The use of LGG at a dose of at least 6 × 108 CFU during a course of antibiotics may prevent AAD, and early high-dose treatment during rotavirus-associated diarrhoea may decrease the duration of disease, the frequency of diarrhoea episodes and aid recovery.
Inflammatory bowel disease
IBD is an umbrella term for a number of different diseases, which includes Crohn’s disease and ulcerative colitis.73 As the name suggests, inflammation plays a major role in its development, and its aetiology is thought to be a combination of both genetic and lifestyle factors.74,75,76 The gut microbiota may also play a major role in the pathogenesis of IBD, and in individuals with Crohn’s disease, gut dysbiosis has been reported with an increased growth of E. coli and a reduction in the bacterial phyla Firmicutes, of which LGG is a member.77,78
As previously discussed, LGG may have pro-inflammatory properties, and limited effects in inflammatory GI diseases such as IBD. Remission of Crohn’s disease, endoscopic recurrences and relapse times have all been shown to remain unaffected by LGG supplementation. One systematic review and meta-analysis of 41 studies, two of which were in LGG, reported no difference in endoscopic recurrences when supplemented with LGG, compared with placebo (0.93; 95% CI: 0.63, 1.38).79 In a second meta-analysis of six RCTs, four of which were in LGG, the supplementation of LGG was concluded to increase the relapse rate of individuals with Crohn’s disease.80 In this trial of 359 individuals, placebo showed a greater benefit on clinical relapse rates in adults (RR = 1.85, 95% CI: 1.00−3.41) and children (RR = 1.68, 95% CI: 1.07−2.64) compared with LGG, with little heterogeneity between the studies (P = 0.71, I2 = 0%).
Studies on the use of LGG in children have also not been efficacious. LGG in combination with standard Crohn’s disease treatment has shown marginally shorter time periods between relapses. One RCT with a 2-year follow-up in 75 children who were in a period of inactive Crohn’s disease reported a non-significant shorter time between the median time to relapse in those treated with LGG compared with those on placebo (9.8 months versus 11 months, P = 0.24).81 There is a possibility that concomitant therapies may be masking the effect of LGG; however, in combination with the previously reviewed studies that have shown no effect of monotherapy, it would suggest that this is not the case.
When LGG is combined with other gut bacteria strains, anti-inflammatory actions have been observed; however, given the previous in vitro and in vivo research, it is likely that the extent of anti-inflammatory effects is due to the gut bacteria strain it has been combined with. One open-label parallel study reported an anti-inflammatory effect with a combination of LGG GR-1 strain and Lactobacillus reuteri in a yoghurt supplement given to 20 participants with Crohn’s disease and ulcerative colitis.82 In this study, LGG dosage of 2 × 107 CFU/ml and 1 × 103 CFU/ml and L. reuteri were associated with increased levels of CD4+ CD25high T-cells (P = 0.007), which are involved in immune regulation, and decreased inflammatory cytokines, tumour necrosis factor-alpha (TNF-α) and IL-12 compared with healthy controls. However, the anti-inflammatory effects observed may be due to the presence of L. reuteri.
Given the in vitro evidence and the supplemental studies, there is very minimal evidence for the use of LGG in inactive Crohn’s disease for endoscopic recurrences, and it may even be detrimental to overall relapse rates. Individuals with Crohn’s disease have been reported to have antibacterial reactivity and a loss of tolerance for their own enteric flora83 and, given the results above showing low colonisation of LGG in the guts of those with Crohn’s disease, could indicate a need to increase supplemental doses above those already tested. However, this would raise safety concerns and, given the lack of dose−response trials, this is an area that requires more research.
Body weight
The relationship between body weight, diet and gut microbiota is complex, with each component influencing the other.84 The involvement of the gut microbiota in the development of metabolic disorders is thought to involve nutrient and lipid metabolism, and hormone and immune modulation. In animal models, diets supplemented with LGG had hypercholesterolaemic effects and caused increased satiety, with increased peptide YY production;85 however, mechanisms are still being debated.
The role of the two major phyla, Firmicutes, to which LGG belongs, and Bacteroidetes in obesity and weight loss has been extensively researched and remains controversial. Observations in obese children and overweight/obese women with metabolic syndrome have shown an increased Firmicutes to Bacteroidetes ratio, compared with their healthy counterparts.86,87,88 Furthermore, a reduction in the Firmicutes to Bacteroidetes ratio has been observed in obese individuals following weight loss.89
The controversy over the role of Firmicutes and LGG occurs when looking at supplementation. Studies would suggest that LGG has little effect on weight gain, as two trials of LGG with differing gut bacteria species have shown differing results. One RCT reported no significant effect of a combination supplement containing 6.5 × 109 CFU/day LGG and B. lactis on the prevention of excessive gestational weight gain in 230 obese pregnant women (probiotics versus placebo, RR = 1.14, 95% CI: 0.99−1.31).90 However, in contrast, one RCT showed that supplementation with a multispecies probiotic of Bifidobacterium animalis and LGG at 1 × 109 CFU/day in 411 obese and overweight pregnant women resulted in lower maternal weight gain compared with placebo.91 It could be concluded from these two studies that the presence of LGG is having no effect on weight loss or gain, and it is the other species of gut bacteria that may be exerting its effects.
Underlying pathophysiology during obesity may also remain unaffected by LGG supplementation. One RCT sub-study of 26 healthy adults reported that LGG supplementation of 6.2 × 107 CFU/day for 3 weeks in a milk-based fruit drink resulted in changes in serum global lipid profiles, with decreased lysophosphatidylcholines (P ≤ 0.05), sphingomyelins (P ≤ 0.001) and several glycophosphatidylcholines (P ≤ 0.05), which may be involved in the pathophysiology of atherosclerosis.92,93,94 It should also be noted that triglycerides were increased and when the trial adjusted their statistics to allow multiple hypothesis analysis, no changes were observed in global lipidomic profiles.
The effect of LGG in weight loss remains controversial. Observational studies have outlined a negative effect of the Firmicutes phyla on weight loss, but supplemental trials of specific strains are not straightforward. As effects may be species dependent, it cannot be discounted that positive trials of supplementing multispecies probiotics containing LGG were due to a symbiotic effect of the two strains or due to the Bifidobacterium strain present in the supplement. It should be noted that caloric intake and exercise were not measured in these trials, which could affect outcomes.
Liver disease
The gut−liver axis is now a well-recognised relationship that is thought to interact through the mesenteric portal vein.95 The pathological progression of non-alcoholic fatty liver disease (NAFLD) development is thought to involve inflammation and lipotoxicity.96 Thus, targeting the gut−liver axis to treat NAFLD may be promising for the treatment of this multi-factorial disease.
The use of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) as clinical markers of liver damage is well established. However, an understanding of liver disease is not limited to liver function, but also considers the presence of small intestinal bacterial overgrowth (SIBO) and intestinal permeability, as endotoxaemia may contribute to reduced life expectancy in individuals with liver cirrhosis.97 The use of probiotics to improve SIBO is thought to occur from successful colonisation of the small intestine, which prevents microbial translocation.98 However, in one RCT of 53 patients with chronic liver disease, successful colonisation of LGG and improved SIBO did not translate into improved intestinal permeability and liver function after 4 weeks.18 This study used a multispecies probiotic of six different strains, including LGG, and reported increased LGG in faeces (P ≤ 0.001) and improved SIBO (P ≤ 0.05), but only marginally improved intestinal permeability and no changes to liver chemistry. Short treatment duration and the study population could be responsible for observations.
In comparison, longer studies on LGG monotherapy in children have reported improved liver chemistry. Compared with placebo, 8 weeks of LGG supplementation (1.2 × 1010 CFU) in 20 children with NAFLD has been associated with decreased ALT (P ≤ 0.03) and anti-peptidoglycan-polysaccharide antibodies (P = 0.03),99 which are polymers from the cell wall of bacteria that may contribute to inflammation in certain chronic inflammatory diseases.100 Differences in dosages between the studies may account for conflicting results; however, the dosage was not disclosed in the previous study. The previous study may also have been too short to observe differences, or the use of a multispecies probiotic may be masking the effects of LGG. The small number of study participants in the monotherapy study may have also been giving a false-positive.
The research surrounding the use of LGG for the improvement of liver function and SIBO is still in its infancy. The use of LGG for the improvement of liver function in paediatric liver disease is promising at a dose of at least 1.2 × 1010 CFU/day. In adults, more large-scale RCTs are required, as there is yet no compelling evidence for LGG efficacy in NAFLD, despite promising results for improving SIBO when given in combination with other species. More research is also required in this area, especially with regards to those with severe disease, as the use of probiotics in immunocompromised individuals has raised some concerns.18
Insulin resistance and type 2 diabetes
Gut dysbiosis has been linked to insulin resistance (IR) and type 2 diabetes (T2D). In contrast to studies on individuals with obesity, individuals with T2D show compositional changes of the intestinal microbiota, which include decreased Firmicutes, resulting in an increased Bacteroidetes to Firmicutes ratio within the intestinal microbiota.101 Despite this, studies on individuals with T2D have reported increased total Lactobacillus anaerobes, with pronounced levels of L. reuteri and Lactobacillus plantarum;102 however, the role of native LGG in those with T2D is unclear.
As previously discussed, gut dysbiosis and the development of intestinal permeability may lead to endotoxaemia and systemic inflammation, and this may contribute to the pathogenesis of IR and T2D. The effects of probiotics in T2D may be through the production of glutathione, decreasing inflammation and oxidative stress. Supplementation of a multispecies probiotic containing LGG for 8 weeks in 54 individuals with T2D was shown in one trial to prevent a rise in fasting plasma glucose, decrease blood markers of inflammation (−777.57 ng/ml versus +878.72 ng/ml, P = 0.02) and increase the antioxidant glutathione compared with placebo (240.63 µmol/l versus −33.46 µmol/l, P = 0.03).103 Measures of IR were increased in both groups; however, less so in the probiotic group (+2.38 versus +0.78, P = 0.03).
As with many studies in multispecies probiotics, it is important to understand the role of LGG to rule out influences from other species. Studies on streptozocin-induced diabetic rats reported improved glucose tolerance and IR after 4 weeks consumption of LGG.104 However, animal studies on LGG dominate, and trials in humans with T2D are lacking. Amongst 200 healthy individuals, LGG supplementation of 1 × 109 CFU for 90 days was shown in one trial to help maintain glycaemic control.105 Compared with placebo, where glycated haemoglobin (HbA1c) increased over the 90 days, the LGG-supplemented group reported sustained HbA1c levels (P = 0.005 between-group comparison), indicating possible attenuation of T2D development in healthy adults.
Results from the study above indicate that supplementation of LGG may be of benefit in slowing the development of T2D, an effect that was also observed in gestational diabetes.106 In this trial of 256 pregnant women with normal glycaemic levels, those supplemented with multispecies LGG + B. lactis in combination with dietary advice reported improved blood glucose control during pregnancy and a reduced risk of elevated glucose concentration compared with placebo [odds ratio (OR) 0.31, 95% CI: 0.12, 0.78, P = 0.028]. These effects were sustained 12 months post-partum; however, dosages were not stated in the trial and the role of a single-strain LGG probiotic is unclear. Moreover, a potential effect of the dietary changes cannot be discounted. In contrast, a multispecies probiotic of LGG + B. animalis (1 × 109 CFU/day) discussed previously in this review failed to prevent gestational diabetes.91 Taken in tandem, these studies would suggest that effects on glycaemic control in pregnancy could be dependent upon dietary changes.
The use of 1 × 109 CFU LGG as monotherapy or as part of a multispecies probiotic to slow the pathophysiological continuum from IR to T2D in healthy individuals may be of benefit. The studies on gestational diabetes mellitus (GDM) would suggest little effect of LGG, and that dietary changes were the driving factor.
Cystic fibrosis
Intestinal inflammation is a predominant feature in adults and children with cystic fibrosis (CF), with levels similar to individuals with IBD.107,108 Improvements to intestinal inflammation in patients with CF have been reported following probiotics109 and LGG supplementation as a monotherapy. The restoration of disrupted intestinal microbiota and improvements to intestinal inflammation in children with CF has been reported following supplementation with LGG.108,110 In one RCT, supplementation of 6 × 109 CFU/day LGG resulted in reduced faecal calprotectin (CLP), which is indicative of intestinal inflammation, in the GI tract of 22 children with CF (184 ± 146 µg/g versus 52 ± 46 µg/g, P ≤ 0.01).110 Correlations between reduced microbial richness and intestinal inflammation were also observed in this trial (r = 0.53, P = 0.018).
Although inflammation may be improved, the effects of LGG supplementation on pulmonary exacerbations and hospital stays remain controversial. LGG supplementation of 6 × 109 CFU/day reduced pulmonary exacerbations and hospital admissions in one 6-month RCT of 43 children with CF; however, the duration of stay did not differ between the groups.111 In a more recent, larger RCT in 95 children, LGG supplementation (6 × 109 CFU/day) failed to show any effect on hospitalisations (OR 1.67, 95% CI: 0.75−3.72, P = 0.211) or exacerbations (OR 0.83, 95% CI: 0.38−1.82, P = 0.643).112 In contrast to the previous trial, this trial of 95 children with CF ran for 12 months. Study design and duration may account for differences, with parallel studies enabling comparisons between treatments at the same time amongst differing individuals, whereas crossover studies negate the effects of between-patient variability. In this instance, the crossover study could eliminate differences between the participants, such as severity of disease or dietary and lifestyle differences.
The data for the use of LGG in CF mainly revolve around studies in children. Supplementation with 6 × 109 CFU LGG may reduce intestinal inflammation and restore gut microbiota eubiosis; however, the exact effects on pulmonary exacerbations remain unclear.
Respiratory tract infections
Removal of the adenoids has been associated with increased long-term risk for respiratory tract infections (RTIs),113 indicating their possible involvement in the body’s immune defence against respiratory diseases. Although largely considered a member of the gut microbiota, the importance of LGG for the immune system may be apparent from its presence in large amounts in the adenoids of children, which has been shown to increase following 3 weeks of supplementation.114
However, its role in the prevention of RTIs and its ability to reduce symptoms and duration of illness remain controversial. The use of an LGG supplement (1 × 109 CFU/day) for 6 weeks in 59 adults artificially infected with human rhinovirus failed to affect viral load when compared with placebo.115 Furthermore, one study failed to show benefits to severity of cold symptoms of a live LGG supplement (1 × 109 CFU/day) compared with an inactivated form and placebo in 60 adults after 6 weeks.116 Although this study did indicate a trend towards lower occurrence and severity of cold symptoms in the active LGG group, this was not significant.
Studies in younger adults and children have shown more beneficial outcomes of LGG supplementation in RTIs. Amongst 231 healthy college students, the use of LGG (1 × 109 CFU/day) in combination with B. animalis for 12 weeks was shown to improve the severity and duration of upper RTIs (URTIs), with a 2-day shorter average infection, leading to fewer school days missed compared with placebo (P = 0.002).117 As this study was in combination with B. animalis, it is difficult to determine the exact effect of LGG; however, the combination therapy was shown to be of benefit.
Over-the-counter RTI medications in children under 6 years old are often avoided and discouraged due to concerns with safety and efficacy,118 indicating a need for alternative therapies in this cohort. Children attending day care facilities are particularly susceptible to RTIs due to factors such as increased exposure to infections119 and cessation of breastfeeding,120 which contributes to a number of childcare and workdays lost.121 Therefore, the use of probiotics may be of benefit. Strain-specific effects have been highlighted, with LGG reducing the duration of RTIs in children at day care, which other strains failed to do in a systematic review and meta-analysis of 15 RCTs with 5121 children (MD 0.78 days, 95% CI: −1.46 to 0.09).122 Interestingly, this meta-analysis reported no effect on incidence, antibiotic use or days missed from day care, which differs to an earlier systematic review and meta-analysis of four RCTs in 1805 children, which reported reduced risk of URTIs (RR = 0.62, 95% CI: 0.50−0.78, NNT = 4) and antibiotic use (RR = 0.80, 95% CI: 0.71−0.91)123 with LGG supplementation. Differences between the results may be due to a lack of recent data supporting LGG supplementation, or could be owing to differing trial designs and outcome measures.
In high-risk children, the supplementation of prebiotics and probiotics may reduce the risk of RTIs and rhinovirus infections. In one study of 94 preterm infants, supplementation with LGG (1 × 109 CFU/day for the first 30 days, and 2 × 109 CFU/day for a further 30 days) reduced the incidence of RTIs (RR = 0.50, 95% CI: 0.28−0.90, P = 0.022) and rhinovirus infections (RR = 0.49, 95% CI: 0.24−1.00, P = 0.051).124 In a second study, LGG supplementation (1 × 109 CFU/day) in 742 hospitalised children reduced the risk of RTIs compared with placebo, but failed to impact hospitalisation duration.125
The apparent lack of efficacy of LGG in adults for the prevention of RTIs may be due to suboptimal dosages, as the trials above largely used dosages like those used in the studies on children. Dosage−response studies are warranted to investigate this. Studies on children have shown positive results for the use of LGG in reducing the duration of RTIs in those attending day care. In high-risk children, LGG may reduce the occurrence of rhinovirus infections when supplemented with at least 1 × 109 CFU/day for at least 2 months.
Otitis media
Usually considered an extension of an URTI in children, otitis media is a spectrum of diseases characterised by middle ear inflammation resulting in pain, irritability and fever.126 As previously discussed, the presence of LGG in tonsil and adenoid tissue is indicative of its role in the immune system and RTIs,114 but there are limited data on its role in otitis media. LGG has been detected in the middle ear following supplementation, but LGG may already be present in the middle ear of children with otitis media and, compared with placebo, LGG supplementation may have limited effects on children undergoing tympanostomy, which is a procedure to prevent fluid build-up in the middle ear.127 In one study of 309 children prone to otitis, a multispecies probiotic containing LGG supplemented (8−9 × 109 CFU/day) for 24 weeks failed to reduce pathogenic bacteria in the nasopharynx, or reduce frequency and occurrence of acute otitis media.128 In contrast, a multispecies probiotic containing LGG supplemented for 6 months reduced the presence of human bocavirus (HBoV), which is the primary pathogen in otitis media, in 269 children prone to otitis (6.4% versus 19.0%, OR 0.25, 95% CI: 0.07−0.94, P = 0.039); however, the effects on symptoms and otitis media were not discussed.129
The presence of LGG in the middle ear may be indicative of its role in infection prevention; however, there are limited data regarding the use of LGG for the management of otitis media in children and adults. Although studies are positive for the use of LGG as part of a multispecies probiotic to reduce the presence of the virus, more studies are warranted on LGG in isolation.
Anxiety and depression
Anxiety and disordered gut function often coexist, with symptoms of nausea and stomach pain reported by individuals with elevated anxiety.130 The gut microbiota may communicate in a bi-directional manner with the brain along the gut−brain axis in a number of different ways, such as signalling through metabolites, through the enteric nervous system and the neural-immune system.131 Under physiological conditions, neurotransmitters such as γ-aminobutyric acid (GABA) may be synthesised and released from Lactobacillus.132 However, under pathophysiological conditions, inflammatory cytokines produced in the gut may also have the capacity to affect the brain and stimulate the release of cortisol, dysregulating the hypothalamic−pituitary−adrenal (HPA) axis leading to initiation of the stress response.133 Furthermore, gut dysbiosis has been implicated in mental health disorders.134 Therefore, modification of the gut microbiota using probiotics may hypothetically provide a novel treatment target for conditions such as anxiety and depression.
Studies on probiotics in anxiety and depression are extensive; however, they may not be translatable to the use of LGG in isolation. The inclusion of LGG in a multispecies probiotic supplemented for 6 weeks in 70 petrochemical workers showed improvements from baseline in general health (16.9 ± 1.8 versus 9.8 ± 1.9, P = 0.001) and depression and anxiety (18.9 ± 3.2 versus 9.4 ± 4.0, P = 0.006), and no effects were seen in the placebo group, or in any of the groups on the HPA axis, as measured by the General Health Questionnaire and the Depression Anxiety and Stress Scale.135 This indicates that effects may be independent of the HPA axis, or that the short study period may have been insufficient for effects on symptoms to be observed.
The use of LGG as a monotherapy may have a beneficial effect on depression in individuals following the occurrence of myocardial infarction (MI). Supplementation with LGG (1.6 × 109 CFU/day) had beneficial effects on depression, oxidative stress and inflammation in individuals post-MI who had undergone percutaneous intervention (PCI).136 This study of 44 individuals reported that, compared with placebo, 12 weeks of supplementation with LGG decreased symptoms of depression (−5.57 versus −0.51, P = 0.045) and increased QoL (23.6 versus 0.44, P = 0.023). Biomarkers for inflammation and oxidative stress were also decreased in the supplementation group compared with placebo. Low-grade inflammation may contribute to the development of depression,137 and the mechanism of action of LGG in depression and inflammatory diseases may be through its immunomodulatory properties.
During pregnancy, physical and psychological changes can occur leading to stress and adverse outcomes in the baby.138 Pregnancy with obesity may increase the risk for the development of depression and anxiety compared with entering pregnancy at a normal weight,139,140,141 and as probiotics are considered safe during pregnancy,142 they may hypothetically provide a treatment option. However, in practice, clinical trials on a combination of LGG + B. lactis failed to improve the mental health of 230 obese pregnant women at 36 weeks of gestation.143 There were no differences between depression scores, and anxiety and physical wellbeing worsened with time.
Studies on the use of LGG in isolation in depression and anxiety are limited, and although plausible mechanisms for its use exist, further studies are warranted in this cohort of individuals. The use of LGG (1.6 × 109 CFU/day) for 12 weeks for the development of depression and anxiety in individuals who are post-MI is promising.
Attention-deficit hyperactivity disorder and Asperger’s syndrome
Attention-deficit hyperactivity disorder (ADHD) is a childhood neurodevelopmental disorder that is present in as many as 3% of children and predominantly in boys.144 Symptoms such as inattention and hyperactivity are hallmark symptoms of ADHD, which are frequently observed in children with Asperger’s syndrome (AS) alongside disordered emotional behaviour.145 As previously discussed, LGG may affect emotional behaviour via the vagus nerve and regulation of GABA in the amygdala and hippocampus areas of the brain,146 which may also be involved in the pathophysiology of mental health disorders.147
Animal studies have indicated a possible benefit to learning and memory following LGG supplementation;148 however, in humans the life stage at which LGG supplementation occurs may be important for favourable outcomes. Pre-natal and post-natal factors have been implicated as risks for ADHD and AS, and intervention with LGG at both stages may impact development later in childhood. Compared with placebo, LGG supplementation (1 × 109 CFU/day) 4 weeks prior to and 6 months after birth reduced the risk of development of ADHD and AS in 75 children, 13 years later (17.1% versus 0%, P = 0.008).149
Studies on the use of LGG in ADHD and AS are limited; however, what does exist is promising for the use of LGG (1 × 109 CFU) during late pregnancy and infancy to reduce the risk of development. With such strong mechanistic links between neurodevelopmental disorders and the use of LGG, studies are warranted to further investigate possible clinical benefits.
Urinary tract infections
UTIs are a commonly occurring condition, which are ordinarily treated with the use of antibiotics.150 However, research would suggest that this practise may be detrimental, due to the development of multi-drug-resistant bacteria.151 Escherichia coli originating from the gut microbiota is thought to be the cause in the majority of cases, and in women it may colonise the vagina, transfer to the urethral opening and ascend to the bladder.152
Experiments in murine models have shown that the LGG-derived effector protein, HM0539, can competitively inhibit the adhesion of E. coli in the GI tract.153 However, one pilot trial of 42 post-menopausal women indicated that although the GI tract is readily colonised by LGG, vaginal swabs show poor adhesion, with only 9.5% of women having colonisation in this area.154 Possibly owing to this, clinical trials on the use of probiotics for the prevention of recurrent UTIs have shown mixed results, with vaginal colonies recurrently transferring to the urethral opening. The use of Lactobacillus spp. was shown in one systematic review and meta-analysis of nine clinical trials to reduce the risk of recurrent UTIs in females (RR = 0.684, 95% CI: 0.438−0.929, P ≤ 0.001); however, different strains showed varying efficacy and LGG was not analysed.152 When administered as a monotherapy, the regular consumption of cranberry juice, but not LGG, was determined to prevent E. coli-derived recurrent UTIs in one 12-month RCT of 150 women.155 In this trial, 39% of women in the LGG group reported recurrent UTIs compared with 16% consuming cranberry juice and 20% of control. Poor colonisation of the periurethral area and consumption only five times per week were determined to be the possible reasons for lack of efficacy. In contrast, a multispecies probiotic containing LGG in 181 children has been reported to be effective at reducing the risk of recurrent UTIs compared with placebo (P = 0.02); however, in individuals who did have a recurrent event, those on probiotics had a shorter duration to recurrence (3.5 months probiotic versus 6.5 months placebo, P = 0.04).156 As this study looked at multispecies probiotics, it is difficult to determine the role of LGG monotherapy in this cohort.
Amongst individuals who have had a spinal cord injury, the risk of recurrent UTIs may be higher due to physiological alterations in the urogenital system.157,158 The daily use of LGG in combination with Bifidobacterium BB12 (7 × 109 CFU) in a 6-month RCT failed to show efficacy in preventing UTIs in 207 people with spinal cord injury compared with placebo.159 It would appear that only through intravesical administration does LGG improve symptoms of UTIs.160
There appears to be little benefit to women in the use of LGG for the prevention of recurrent UTIs possibly due to poor colonisation in the vaginal area in the absence of the pili that aid adhesion in the GI tract, and continual transfer to the urinary tract.16 Amongst individuals with recurrent UTIs due to spinal cord injury, the use of LGG may only be of benefit to symptoms through intravesical administration. More studies are required in children to determine the role of LGG as a monotherapy, as its inclusion in a multispecies probiotic is promising for the prevention of UTIs.
Infant health
The gut microbiome begins to develop immediately after birth, and can be determined by mode of delivery and feeding.161,162 Infants born vaginally are typically colonised with beneficial bacteria from the mother’s vaginal canal, and those born through Caesarean-section (C-section), from the mother’s skin.163 Individuals born via C-section may have a higher risk of developing several metabolic and immune disorders later in life,164 possibly due to a lack of Escherichia-Shigella and Bacteroides species, and lower bacterial richness and diversity.165
Colonisation of pathogenic bacteria early in life has been shown to contribute to poorer health outcomes later in life.166 However, despite the ability of LGG to competitively inhibit pathogenic bacteria and act as an antimicrobial in adults, results have been mixed in children, with one RCT reporting no effect of 42 weeks of LGG supplementation on the colonisation of Staphylococci in 60 pre-term infants, despite rapid colonisation of LGG.167 In addition, no effects were seen on growth rate or length of hospitalisation in this trial. The analysis of only one pathogenic bacteria may not be sufficient, and other strains may need to be analysed to understand the exact effects. In contrast, benefits to height and weight of babies at 12 months have been observed following in utero LGG supplementation.168 In this RCT, 208 healthy pregnant women were given LGG (7 × 108 CFU/day) in combination with B. lactis (7 × 108 CFU/day), resulting in increased baby weight and height at 12 months compared with placebo. Furthermore, one 6-month RCT of 120 healthy infants fed with LGG (dosage not stated) in supplemented formula have reported better LGG colonisation (91% versus 76%, P ≤ 0.05), which led to better growth compared with formula milk without LGG. Higher than normal defecation was reported in the LGG-supplemented group; however, this was not considered to be diarrhoea or detrimental to health.169 Differing trial durations, stages of supplementation and follow-up times could be responsible for differences between the outcomes, with at least 6 months of treatment required to show benefits.
Observational studies have indicated that maternal nutrition and the in utero environment may also increase the risk of offspring having poor health outcomes,170 indicating an area where probiotic supplementation may be of benefit. Better health outcomes of mothers and babies have been reported in one 2-year follow-up of a RCT, concluding that pre-natal multispecies probiotic use may be a safe and cost-effective way of preventing metabolic disease in offspring.171 In this study, the use of LGG and B. lactis (1 × 1010 CFU/day) in combination with dietary advice in 256 pregnant women from the first trimester to cessation of breastfeeding reduced the frequency of GDM compared with dietary advice alone (P ≤ 0.003).
In those who did develop GDM, smaller birth weight of babies was observed. With birth size being a risk factor for obesity in later life, LGG supplementation may have an impact in utero on later development of non-communicable diseases.
Supplementing LGG in utero or during infancy for improved outcomes at all stages of life is apparent. Although supplementation for improved growth rates in babies is controversial, the results would suggest that 6 months of supplementation either starting in utero or during infancy may be required at doses of at least 7 × 108 CFU/day, and potentially in combination with B. lactis. Supplementing LGG (1 × 109 CFU) in combination with B. lactis (1 × 109 CFU) during pregnancy may have benefits to both the mother and child in preventing the development of GDM and non-communicable diseases in later life.
Infantile colic
Although not life threatening, the impact of a child with colic extends to parental distress, anxiety and depression, and may be associated with the development of disorders such as allergic disease, migraine and GI disorders later in life.172 Thus, the reduction in the time a child with colic spends crying may have huge neuropsychological implications. One recent RCT of 45 colicky breastfed infants showed that a high dose of LGG (5 × 109 CFU/day) in combination with elimination of cow’s milk from the mother’s diet reduced crying time and GI inflammation, with no adverse events reported even at this high dose.173 However, a lower-dose LGG supplementation (1 × 109 CFU/day) for 6 months in an RCT of 184 infants was shown not to prevent colic based on symptoms or physician’s diagnosis when compared with control.174 This finding was supported in an earlier pilot study of 17 breastfed infants given LGG (4.5 × 109 CFU/day) in combination with behavioural support and cow’s milk elimination by the mother.172 LGG supplementation did not affect crying time or GI inflammation, but crying occurrences were decreased. Differing dosages used in the trials above may account for discrepancies between the results, with a higher dosage being more successful. Elimination of cow’s milk may also account for discrepancies.
LGG when included as part of a multispecies regimen has shown more consistent success. When included as part of a nine-strain multispecies synbiotic, a recent RCT of 4 weeks in 17 breastfed infants reported efficacy, with a decrease in the number of crying days and average crying duration when compared with Simethicone, which is used to relieve gas and GI discomfort.175 Although this trial was small, these findings were also supported in a larger, earlier RCT of 50 breastfed infants with higher treatment success and higher symptom resolution when given a seven-species synbiotic containing LGG (1 × 109 CFU/day).176
The use of high-dose LGG supplementation as monotherapy (5 × 109 CFU/day) in combination with cow’s milk elimination has been shown to be efficacious in colic to reduce crying time and GI inflammation. Furthermore, when included as part of a multispecies synbiotic regimen, LGG may be of benefit to infants with colic to improve symptoms and crying. However, there are no studies to date showing efficacy of the use of LGG as a monotherapy.
Human immunodeficiency virus
As with colorectal cancer, the success of treatments for human immunodeficiency virus (HIV) is often dependent upon their tolerability. Diarrhoea is a common side-effect of anti-retroviral treatments177 and, in addition, patients who are immunocompromised may be at a higher risk of microbe-associated diarrhoea.178 However, unlike patients with colorectal cancer, 17 patients infected with HIV who had suffered from diarrhoea for more than 1 month showed little improvement to diarrhoea or GI symptoms following LGG supplementation twice daily (1−5 × 1010 CFU) for 2 weeks compared with placebo.179 There were no differences in faecal counts of LGG between the two treatments, indicating poor colonisation following supplementation.
Previous trials have shown lower faecal Lactobacillus cultures in patients infected with HIV compared with healthy individuals,180 indicating a possible need for increased dosages. Further trials are warranted, as the use of LGG in other cohorts of patients for the prevention and treatment of diarrhoea has been of benefit.
Acne
The pathogenesis of acne involves several factors, including inflammation and alterations of insulin signalling.181,182 Systemic supplementation of probiotics to improve the insulin signalling pathway has been discussed previously, and hypothetically LGG could be used to improve acne. Improvements to the expression of genes involved in the insulin signalling pathway of individuals with acne have been reported with supplementation of L. rhamnosus SP1 (3 × 109 CFU/day) for 12 weeks.183 Subjects (n = 10) in the supplementary arm reported reductions in IGF-1 gene expression (P ≤ 0.001) and increased FOXO1 gene expression (P ≤ 0.001) from baseline, with no changes observed in the placebo arm (n = 10). This resulted in physician-rated improvements to skin appearance of acne in the L. rhamnosus SP1 group compared with the placebo group (OR 28.4, 95% CI: 2.2−411.1, P ≤ 0.05). Although the study states that the strain used is also known as LGG, there is very little research to confirm this; however, based on the previous research on LGG and IR, it would appear it may have similar actions. It should also be noted that the study was only completed in Caucasian subjects, and translatability into the skin of other races is unknown. In addition, small sample sizes and the pilot nature of the study warrant further research.
Based on a single, small pilot study, the use of L. rhamnosus SP1 (3 × 109 CFU/day) for at least 12 weeks for the improvement of acne through modulation of the insulin signalling pathway is promising; however, more research is needed in larger higher-powered studies to confirm effects. Studies on the genetic sequencing of L. rhamnosus SP1 and its relationship to LGG or further research on LGG as the test probiotic are also warranted.
Allergy
Allergy development is thought to involve both genetic and environmental factors.184,185 Dysbiosis and reduced diversity of the infant gut microbiome are thought to be included in the pathogenesis of allergic disease in children, due to factors such as antibiotic use in utero and birth by C-section.186 However, effects may be ameliorated using probiotics. One RCT on the use of a multispecies probiotic containing LGG (5 × 109 CFU/day) during pregnancy, breastfeeding and infancy, reported altered effects of antibiotics and C-section birth on gut dysbiosis, increasing Bifidobacteria and reducing pathogenic Proteobacteria and Clostridia,186 indicating that the use of probiotic supplementation during infancy may help to restore eubiosis.
Observational studies have indicated that the involvement of Lactobacillus species may be of particular importance in the development of allergies. The presence of LGG, Lactobacillus casei and Lactobacillus paracasei early in life is associated with lower prevalence of allergic disease in childhood, and there may be a lower presence of Lactobacillus in children with a genetic predisposition, due to one or more parent having allergic disease.187,188
The use of LGG supplementation to decrease the risk of allergy development has also been studied. Benefits to the prevalence of allergic disease later in life were apparent in a follow-up of patients from four separate RCTs on 303 pre-term children given different strains of probiotic.189 Children who were given LGG perinatally had a decreased prevalence of allergic disease compared with children given placebo at the 2-year follow-up (OR 0.62, 95% CI: 0.38−0.99, P = 0.047). Treatment durations from the four included trials ranged from 3 to 6 months, and dosages from 1 × 109 to 5 × 1010 CFU.
In children who have already developed an allergy such as cow’s milk allergy (CMA), LGG supplementation may also be of benefit. One recent systematic review and meta-analysis of 10 studies on LGG (1.4 × 107−5 × 109 CFU/day) reported that supplementation may aid recovery from GI symptoms, promote tolerance to the allergen and improve faecal blood.190 Evidence was rated as low-to-moderate quality, due to issues with blinding, concealment and unclear data; however, studies were RCTs. Tolerance acquisition following LGG supplementation in infants with IGE-mediated CMA may be due to its ability to influence gut microbiota structure, enabling colonisation of Oscillospira.191 The modulation of epigenetic mechanisms involved in the immune system and pathogenesis of CMA may also occur following LGG supplementation, resulting in increased tolerance to cow’s milk.192
Immunomodulation by LGG has also been observed in adults with birch pollen allergy and oral allergy syndrome.193 This RCT of 38 patients received LGG (2 × 1010 CFU/day) for 5.5 months starting 2.5 months prior to allergy season resulting in increased allergen-specific immunoglobulin (Ig)A levels compared with baseline, effects that were not seen with placebo. This may be of benefit to symptoms, as IgA acts to prevent infections and maintain gut microbiota homeostasis, which if disrupted has been associated with an elevated risk of allergies in children.184 A second RCT on the effects of LGG supplementation (3 × 108 CFU/day) for 3 months on allergy in 141 marathon runners reported no effect on the immune marker IgE or several other allergic inflammatory markers, compared with placebo.194 Suboptimal dosages could be responsible for the lack of immunomodulatory effects in this trial, or the fact that the trial only looked at the inflammation-associated IgE and not the anti-inflammatory IgA. Although no symptom relief was observed in these trials, it is indicative of further immune effects in adults on IgA.
There is extensive clinical research on the efficacy and mechanisms behind the use of LGG to prevent allergic diseases and improve symptoms of CMA in children. Children at a high risk of developing allergic disease due to genetic predisposition, antibiotic use or C-section birth may benefit from at least 1 × 109−5 × 109 CFU/day for at least 3−6 months. Children with existing CMA may benefit from 1.4 × 107−5 × 109 CFU/day for at least 4 weeks and up to 3 years. Dosages of 2 × 1010 CFU LGG may be of benefit to adults with birch pollen allergy for immunomodulation and the promotion of IgA. However, further studies are warranted to determine the significance of immunomodulation, as without effects on symptoms, supplementation may be pointless.
Dermatitis and eczema
Atopic dermatitis (ADe) is the most common chronic skin condition, affecting up to 20% of children and 3% of adults worldwide.195 Pathophysiology of ADe is not fully understood; however, dysbiosis may be involved, as individuals with ADe have lower diversity and levels of Bifidobacterium and Actinobacteria, and higher Staphylococcus than healthy subjects.196,197 Furthermore, studies indicate that like other atopic diseases, gut dysbiosis may contribute to ADe development through immunomodulation.197
The effects of LGG supplementation on the immune system, as seen in patients with allergic disease, indicate a potential for its use in individuals with ADe. An early systematic review and meta-analysis of five RCTs with 889 subjects concluded that LGG was ineffective for the primary prevention of eczema in children, when given both prenatally and postnatally.198 Dosages ranged from 1 × 109 to 1.8 × 109 CFU per day, and the quality of data was good.
When looking at reduction of symptoms, recent RCTs not included in the above meta-analysis have shown differing results. Intrinsic microbiota at early infancy may affect outcomes, and infants with ADe who have higher levels of Bifidobacterium dentium have been shown to not respond to probiotic intervention, compared with those without disease.199 One RCT of 67 children with ADe concluded that LGG as the supplement ComProbi (350 mg) in combination with corticosteroid use was effective at decreasing symptoms of ADe after 8 weeks compared with placebo and corticosteroids (P = 0.014), based on Scoring of Atopic Dermatitis (SCORAD).200 However, it is difficult to determine that effects were due to LGG because corticosteroids were also being used. In a second RCT in 102 infants aged 3−12 months with ADe where corticosteroids were not used as the treatment, but were not precluded during the trial if individuals wanted to use them, no therapeutic effect of LGG based on SCORAD compared with placebo after 12 weeks was reported.201 Results from these two trials would suggest that corticosteroids and not LGG may account for the favourable outcomes.
As part of a multispecies therapy, LGG has shown more consistent results. When combined, LGG and B. animalis were shown in one systematic review and meta-analysis of 21 RCTs on various multispecies combinations to reduce the risk of ADe compared with placebo when administered in utero and during infancy.202 Furthermore, in a recent RCT of 290 children not included in the previous meta-analysis, the administration of LGG + B. animalis (1 × 109 CFU/day) in late infancy for 6 months prevented the development of eczema,203 indicating that the use of LGG as part of a multispecies regime with B. animalis may be of benefit for the prevention of ADe and eczema.
The use of LGG for the primary prevention of ADe and eczema may be of benefit when used as part of a multispecies regime in combination with B. animalis, at a dose of 1 × 109 CFU/day, for at least 6 months. While there is yet no strong evidence for LGG alone, stratification of patients with ADe according to intrinsic microbiota may be of benefit for the improvement of symptoms following LGG use; however, more studies are required. The role of LGG in combination with corticosteroids also warrants more research.
Wounds
The role of skin microbiota in wound healing is well documented, with both skin barrier function and the immune response reported to be microbially mediated.204 Topical application of probiotics for the treatment of burns has shown positive results;205,206 however, oral probiotic supplementation lacks research. It has been hypothesised that the gut microbiota communicates with the skin microbiota in a bi-directional manner through the gut−skin axis, evidenced by cutaneous manifestations following GI disorders.207 Oral LGG supplementation may have the potential to help treat certain skin diseases such as ADe and acne as documented above, therefore there may be potential for it to be of benefit to wound healing. The reduction of infections at incision sites in patients with cancer, detailed previously,3 indicates a benefit of LGG supplementation as part of a multispecies probiotic to aid postoperative healing. However, research on the effects in 20 burn victims found only a modest, non-significant improvement in the time taken to complete wound healing, and no improvements to other clinical outcomes.208
There is no evidence for the use of LGG in combination with other probiotic strains for improvements to postoperative wounds. Research is lacking on monotherapy, and has found little effect on healing time in burn victims.
Dental caries
The presence of Streptococcus and Lactobacillus spp. in the oral cavity has been associated with the presence and onset of dental decay.209 However, as previously discussed, LGG may have species-specific properties and produce an inhibitory microcin-like substance, which has the ability to inhibit bacteria such as Streptococcus.32 In vitro studies have indicated that the consumption of an LGG probiotic may also be able to colonise the oral cavity and inhibit Streptococcus sobrinus.31,210 This may translate into a reduction in the risk of the development of caries. In one RCT, 594 children aged 1−6 years were given milk containing LGG (5−10 × 105 CFU/ml) 5 days a week for 7 months, and showed a reduced risk of the development of dental caries (OR 0.56, 95% CI: 0.36−0.88, P = 0.01), based upon Streptococcus levels from dental plaque and saliva, and the presence of dental caries.211
Colonisation of the oral cavity may be affected by a lack of pili expression.16 There is only one trial on the use of LGG for the prevention of dental caries, as detailed above,211 and more studies are warranted given the mechanistic data. However, the trial that does exist was in many individuals over a relatively long period of time. It may therefore be of benefit to reduce the risk of dental caries in children and young adults. Dosages of at least 5−10 × 105 CFU may be needed in children.
Vaccine adjuvant
The recent COVID-19 pandemic and ability of SARS-CoV-2 to mutate has highlighted a need to improve immune response following vaccination. Orally ingested LGG may modulate the immune system in response to bacteria and viruses involved in the development of diseases. Research in mice given oral Lactobacillus has reported enhanced innate immune response following influenza virus challenge, with increased influenza-specific IgG antibodies and greater protection. RCTs have indicated that LGG may be a useful adjuvant for the immune response following influenza vaccine. One RCT in 42 healthy adults reported increased protection to the H3N2 influenza strain whilst supplementing LGG (1 × 1010 CFU) and inulin for 28 days following vaccination.212 However, in the same study, no differences in seroprotection to the H1N1 or B influenza strains were observed.
Individuals with type 1 diabetes (T1D) are at increased risk of infections,213 and influenza vaccine is recommended; however, whether influenza vaccines are truly successful in this cohort is still being debated.214 Adjuvants to increase the immunogenicity of the influenza vaccine may be important, and use of LGG (1 × 109 CFU) 3 months pre- and post-influenza vaccination in 64 paediatric patients with T1D reduced the inflammatory immune response associated with T1D, decreasing IL-17, IFN-γ, IL-6 and TNF-α, without affecting the seroprotective antibodies, which are needed for effective vaccination.215 However, although antibody-mediated immunity remained unaffected in this trial, the mediation of the inflammatory response may be important for individuals who suffer from autoimmune diseases such as T1D.
Studies on different types of vaccinations and studies on LGG as an adjuvant to the polio, rotavirus, Hib, diphtheria and tetanus vaccinations have been completed with varying success. One RCT of 66 healthy males reported that the use of LGG (1 × 1010 CFU), as an adjuvant to the polio vaccine, nearly doubled the increase of polio-specific IgG antibodies and significantly increased IgA antibodies, compared with placebo.216 A second RCT of 98 pregnant women given LGG (5 × 109 CFU) resulted in more frequent occurrence of higher Hib antibody concentrations following vaccination with Hib, diphtheria and tetanus in the offspring; however, IgG remained unaffected. In contrast, LGG supplementation (1 × 1010 CFU) marginally but not significantly improved rotavirus antibodies following vaccination in 620 infants.217 This may correspond to the findings above regarding LGG competitively inhibiting and acting as an antimicrobial against rotavirus, which could prevent the body from becoming infected and building an enhanced immune response when the body is faced with the live rotavirus as part of a vaccine.
The use of LGG supplementation (at least 1 × 109 CFU) as part of a vaccine adjuvant has been shown to be of benefit to the success of the response of biomarkers to vaccines, but only following influenza H3N2, polio and Hib. Further research needs to be performed with other vaccinations to determine effects.
Safety
Probiotics belonging to the genus Lactobacillus and Bifidobacterium are generally regarded as safe (GRAS) by the United States Food and Drugs Administration (FDA).218 However, some studies on Lactobacillus have reported bacteraemia in specific populations, primarily amongst immunocompromised paediatric patients.219 In adults, incidences of bacteraemia-associated endocarditis, primarily in those with a structural heart defect, have also been reported.220 A recent systematic review has indicated that LGG may increase the risk of complications in patients who are immunocompromised, who have critical illnesses, structural heart disease or who have a central venous catheter.69 In pregnancy and lactation, a recent meta-analysis and systematic review concluded that probiotics are safe for use during pregnancy and lactation. Data from the trials included in this review showed that adverse events in pregnancy and lactation following LGG supplementation were minor, and one systematic review and meta-analysis has concluded that probiotic use is safe during pregnancy and lactation;142 however, it would still be recommended to consult with a doctor prior to commencement.
Drug−nutrient interactions are very few, with minor warnings whilst on anti-diabetes drugs due to potential hypoglycaemia and moderate interactions whilst taking antibiotic drugs, as LGG efficacy may be reduced.221
Conclusion
The unique morphological features of LGG may ensure that it has some use as an oral supplement in the reduction of risk of developing ADHD and GDM, in the prevention of allergies and dental caries, for improving immune reactions following vaccines, and for the management of diarrhoea associated with cancer treatments and antibiotic use. Three ways in which it may do this are through immunomodulation, cell growth and proliferation, and as an antimicrobial, aiding it to promote eubiosis. This results in LGG acting to prevent disease development, help manage symptoms and improve underlying pathology. The presence of pili on the exterior aid its colonisation of the GI tract, and its lack of efficacy in disease areas such as UTIs may be due to a lack of expression of these features in certain areas of the body. Effects may be systemic if there is a pathway through which LGG or its products can travel, like the gut−brain axis. However, effects may also be localised and specific, if a transmission pathway does not exist, as seen with its success only in specific cancer types, and diarrhoea treatment in colorectal cancer but not HIV.
There are, however, limitations of this study, and the inability to address genetic variation amongst LGG is apparent. Genetic variants have been found within the LGG species resulting in variations that do not have the spaCBA gene.222 These variants may lack the ability to express the pili-like projections responsible for many of the physiological effects attributed to LGG. It is therefore difficult to exclude the possibility that positive or negative results were not attributable to within-strain differences. Until the research is performed, it is difficult for practitioners to determine which commercial products may have genetic variations. It may be that quality assurance legislation needs to be put in place; however, this has yet to be enacted. A second limitation is that this study could not account for individual intrinsic gut microbiota populations, which are highly personalised.67 Therefore, although patients or disease areas may have been identified to benefit from LGG supplementation, differences between intrinsic gut microbiota may affect efficacy. Amongst the studies, issues with small sample sizes, contradictory results and the fact that the large majority of the research involved the use of multispecies supplements, and the use of other treatments and therapies amongst some of the research, means that conclusions need to be interpreted with caution.
Appendix
Cancer
CFU, colony-forming units; CI, confidence interval; GI, gastrointestinal; IFN-γ, interferon-gamma; IL, interleukin; LGG, Lactobacillus rhamnosus GG; NKCs, natural killer cells; RCT, randomised-controlled trial; RR, relative risk.
Irritable bowel syndrome
CFU, colony-forming units; FAPD, functional abdominal pain disorders; FODMAP, fermentable oligosaccharides, disaccharides, monosaccharides and polyols; GI, gastrointestinal; IBS, irritable bowel syndrome; IBS-A, IBS-mixed subtype; IBS-D, IBS-diarrhoeal subtype; IBS-SSS, IBS-Severity Scoring System; LGG, Lactobacillus rhamnosus GG; RCT, randomised-controlled trial; VAS, Visual Analogue Scale.
Diarrhoea
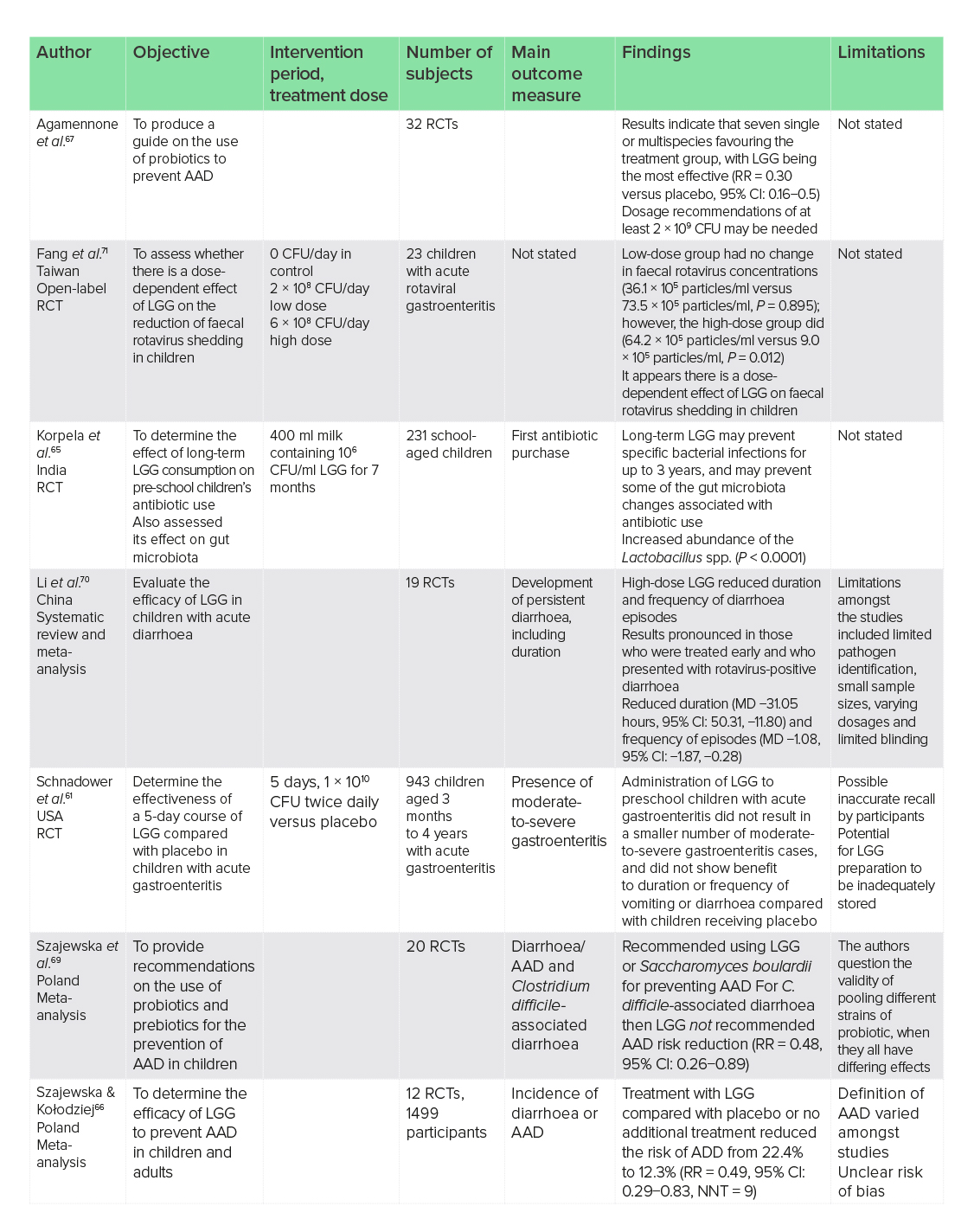
AAD, antibiotic-associated diarrhoea; CFU, colony-forming units; CI, confidence interval; LGG, Lactobacillus rhamnosus GG; MD, mean difference; NNT, number needed to treat; RCT, randomised-controlled trial; RR, relative risk.
Inflammatory bowel disease
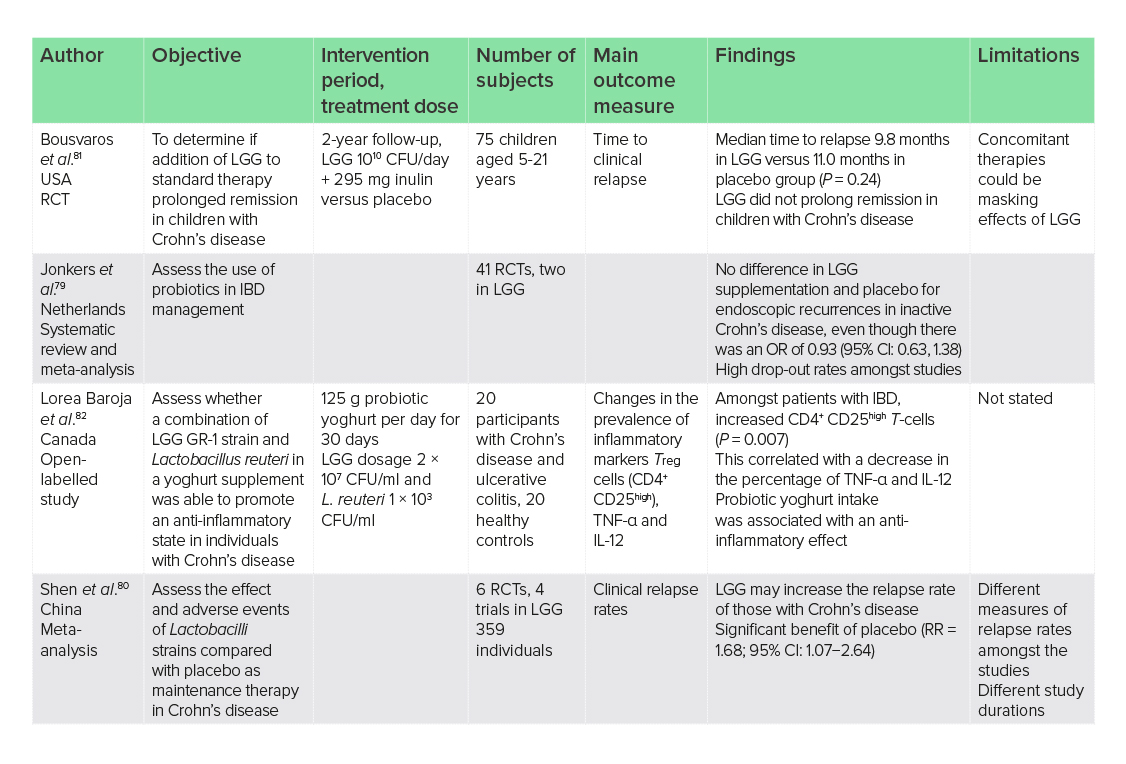
CFU, colony-forming units; CI, confidence interval; IBD, inflammatory bowel disease; IL, interleukin; LGG, Lactobacillus rhamnosus GG; OR, odds ratio; RCT, randomised-controlled trial; RR, relative risk; TNF-α, tumour necrosis factor-alpha.
Body weight
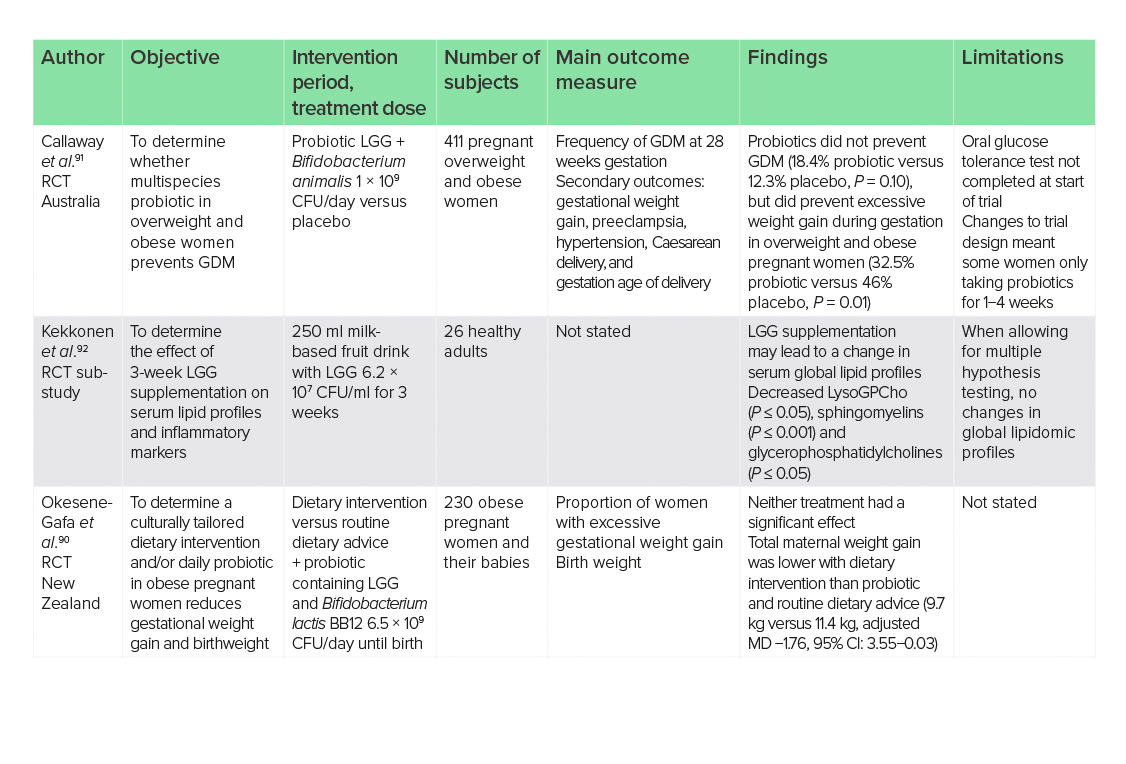
CFU, colony-forming units; CI, confidence interval; GDM, gestational diabetes mellitus; LGG, Lactobacillus rhamnosus GG; MD, mean difference; RCT, randomised-controlled trial.
Liver disease
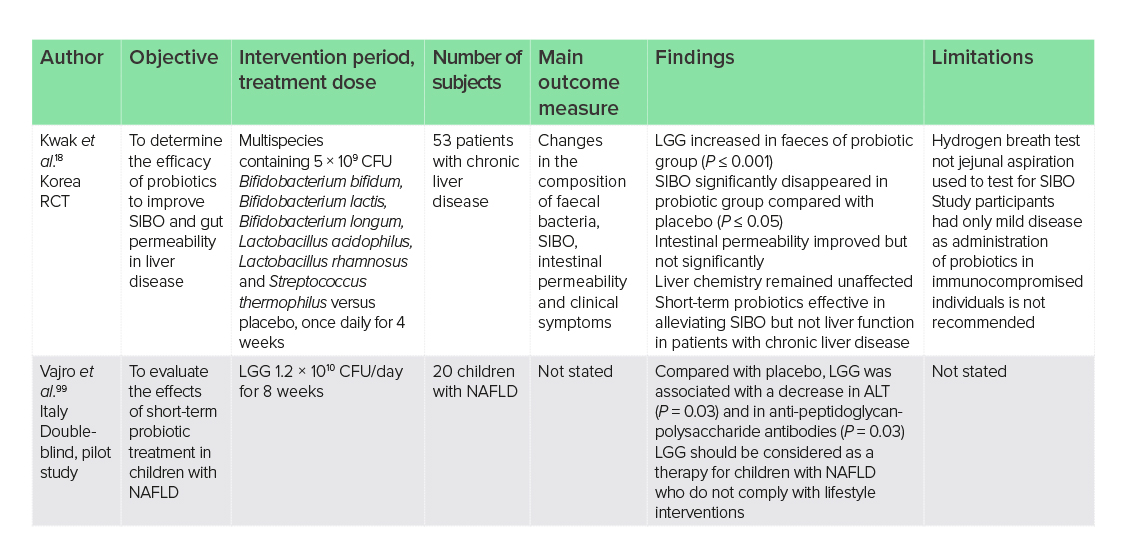
ALT, alanine aminotransferase; CFU, colony-forming units; LGG, Lactobacillus rhamnosus GG; NAFLD, non-alcoholic fatty liver disease; RCT, randomised-controlled trial; SIBO, small intestinal bacterial overgrowth.
Insulin resistance and type 2 diabetes
CFU, colony-forming units; CI, confidence interval; GSH, glutathione; HbA1c, glycated haemoglobin; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; hs-CRP, high-sensitivity C-reactive protein; IR, insulin resistance; LGG, Lactobacillus rhamnosus GG; OR, odds ratio; QUICKI, Quantitative Insulin-Sensitivity Check Index; RCT, randomised-controlled trial.
Cystic fibrosis
CF, cystic fibrosis; CFU, colony-forming units; CI, confidence interval; CLP, calprotectin; FEV1, forced expiratory volume; IBD, inflammatory bowel disease; LGG, Lactobacillus rhamnosus GG; MD, mean difference; OR, odds ratio; RCT, randomised-controlled trial.
Respiratory tract infections
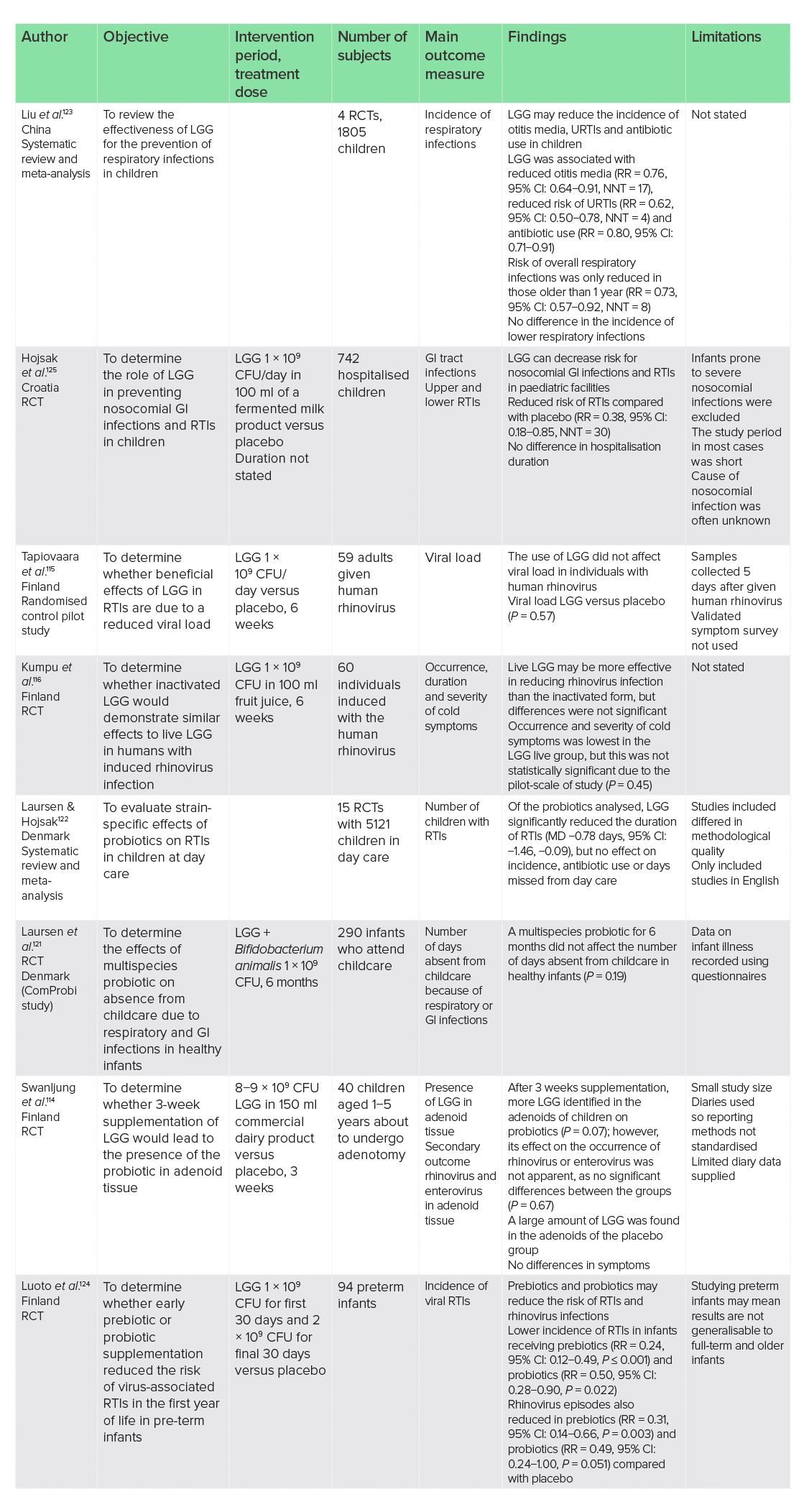
CFU, colony-forming units; CI, confidence interval; GI, gastrointestinal; LGG, Lactobacillus rhamnosus GG; MD, mean difference; NNT, number needed to treat; RCT, randomised-controlled trial; RR, relative risk; RTI, respiratory tract infection; URTI, upper respiratory tract infection.
Otitis media
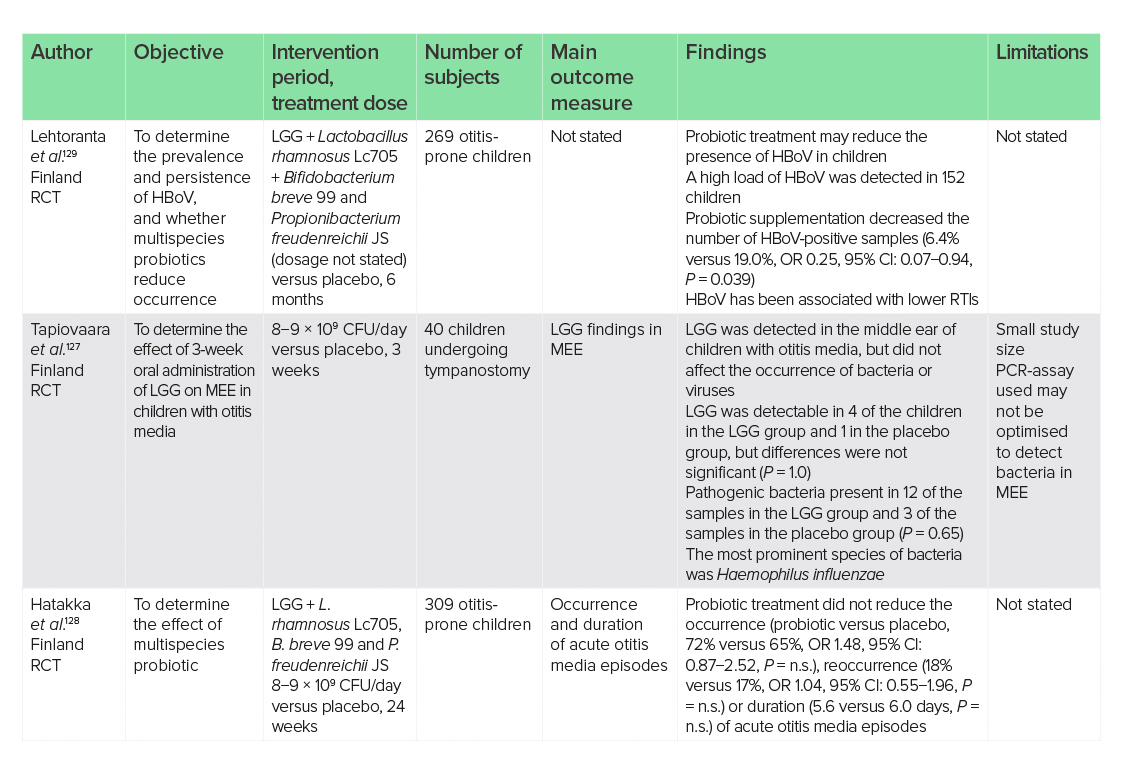
CFU, colony-forming units; CI, confidence interval; HBoV, human bocavirus; LGG, Lactobacillus rhamnosus GG; MEE, middle ear effusion; OR, odds ratio; PCR, polymerase chain reaction; RCT, randomised-controlled trial; RTI, respiratory tract infection.
Anxiety and depression
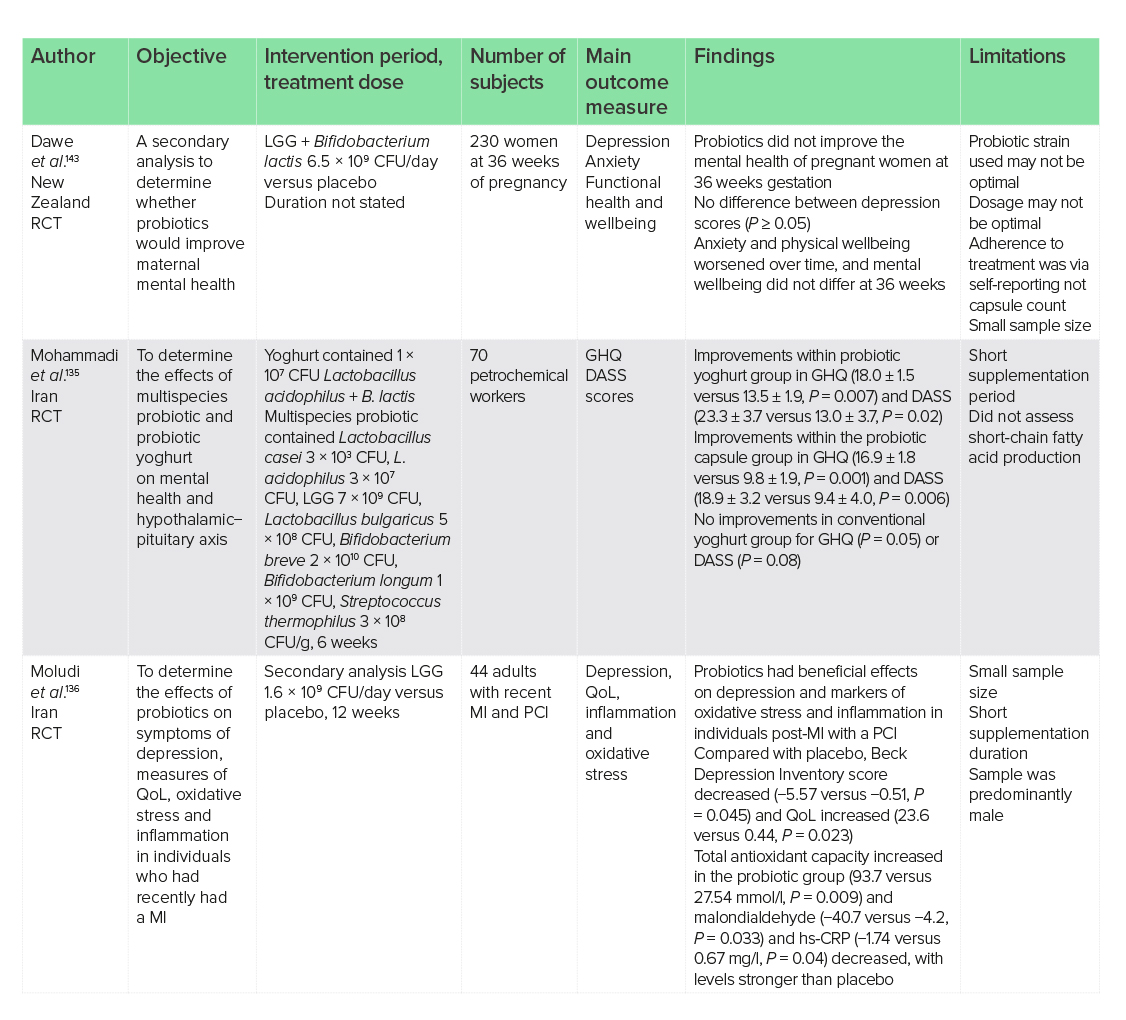
CFU, colony-forming units; DASS, Depression, Anxiety, and Stress Scale scores; GHQ, General Health Questionnaire; hs-CRP, high-sensitivity C-reactive protein; LGG, Lactobacillus rhamnosus GG; MI, myocardial infarction; PCI, percutaneous intervention; QoL, quality of life; RCT, randomised-controlled trial.
Attention-deficit hyperactivity disorder and Asperger’s syndrome
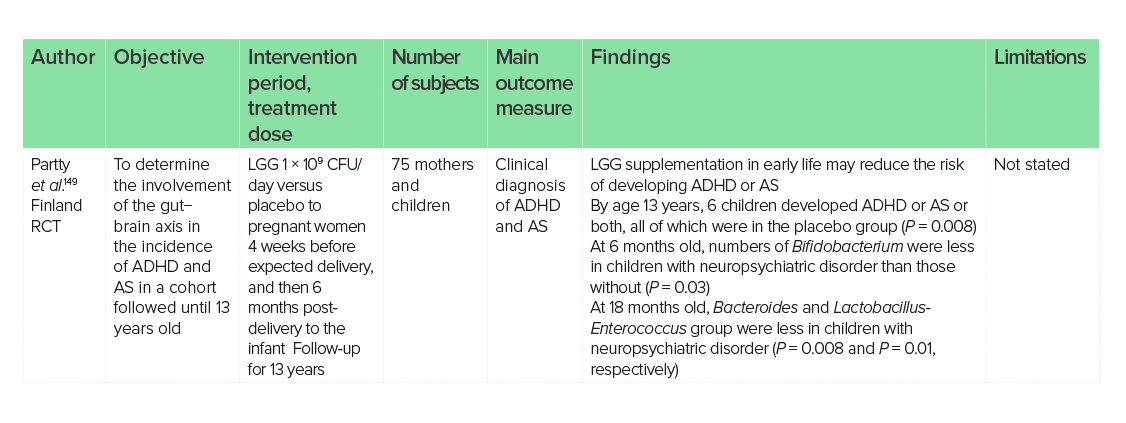
ADHD, attention-deficit hyperactivity disorder; AS, Asperger’s syndrome; CFU, colony-forming units; LGG, Lactobacillus rhamnosus GG; RCT, randomised-controlled trial.
Urinary tract infections
CFU, colony-forming units; CI, confidence interval; GI, gastrointestinal; HR, hazard ratio; LGG, Lactobacillus rhamnosus GG; NNT, number needed to treat; RCT, randomised-controlled trial; RR, relative risk; USQNB-IC, Urinary Symptom Questionnaire for Neurogenic Bladder-IC; UTI, urinary tract infection.
Infant health
CFU, colony-forming units; CI, confidence interval; LGG, Lactobacillus rhamnosus GG; OR, odds ratio; RCT, randomised-controlled trial.
Infantile colic
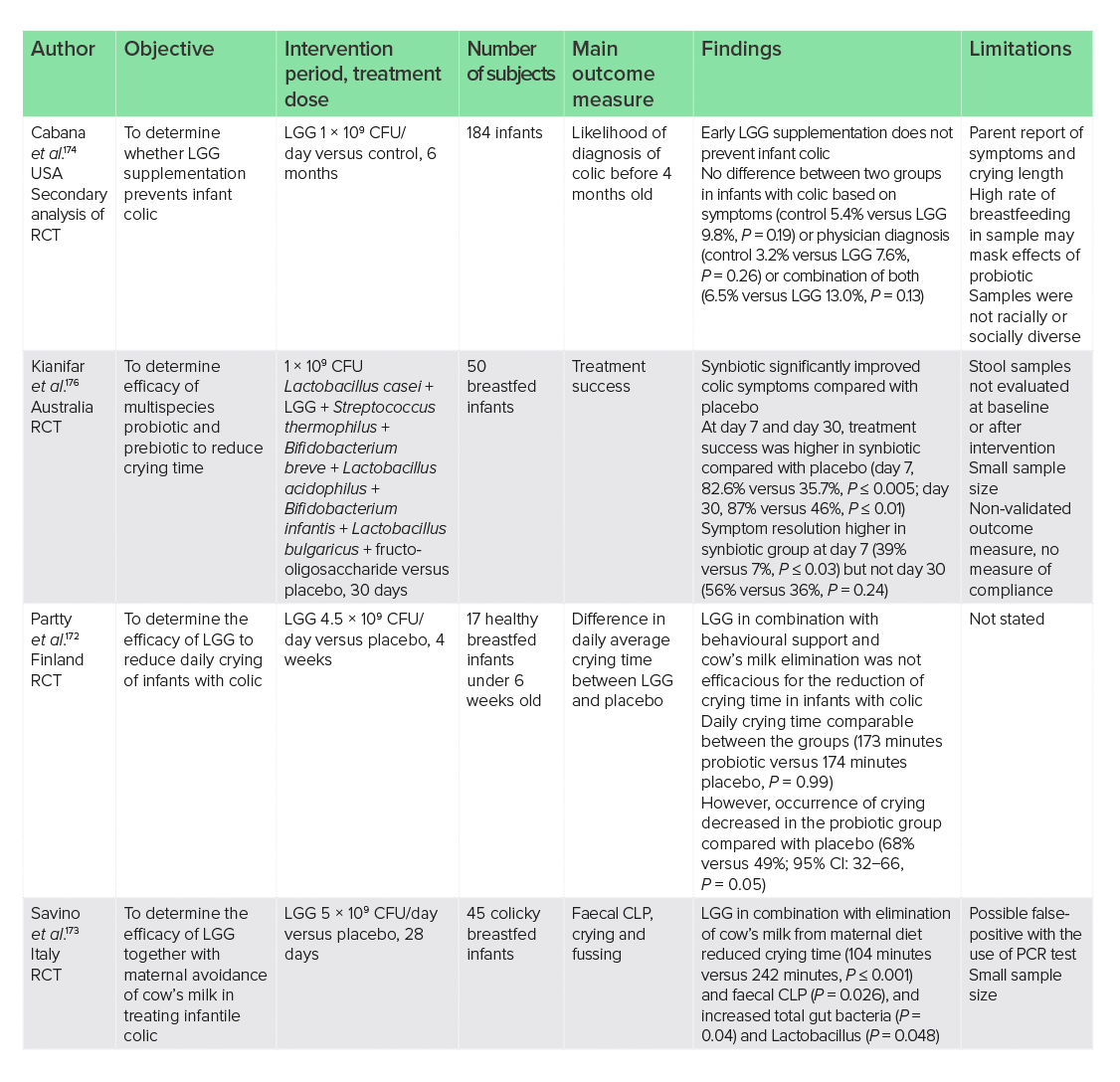
CFU, colony-forming units; CI, confidence interval; CLP, calprotectin; LGG, Lactobacillus rhamnosus GG; PCR, polymerase chain reaction; RCT, randomised-controlled trial.
Human immunodeficiency virus
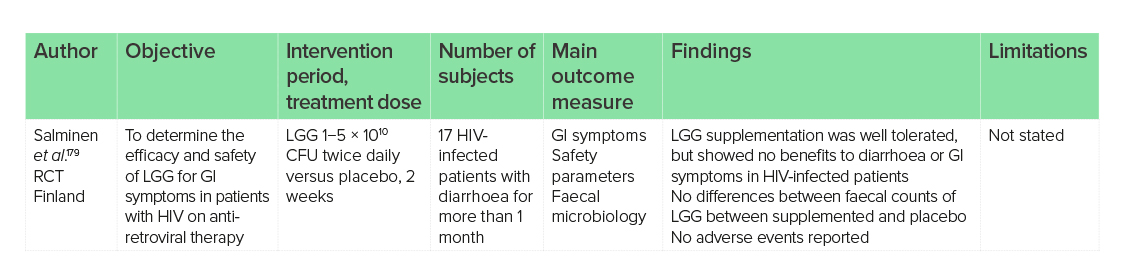
CFU, colony-forming units; GI, gastrointestinal; HIV, human immunodeficiency virus; LGG, Lactobacillus rhamnosus GG; RCT, randomised-controlled trial.
Allergy
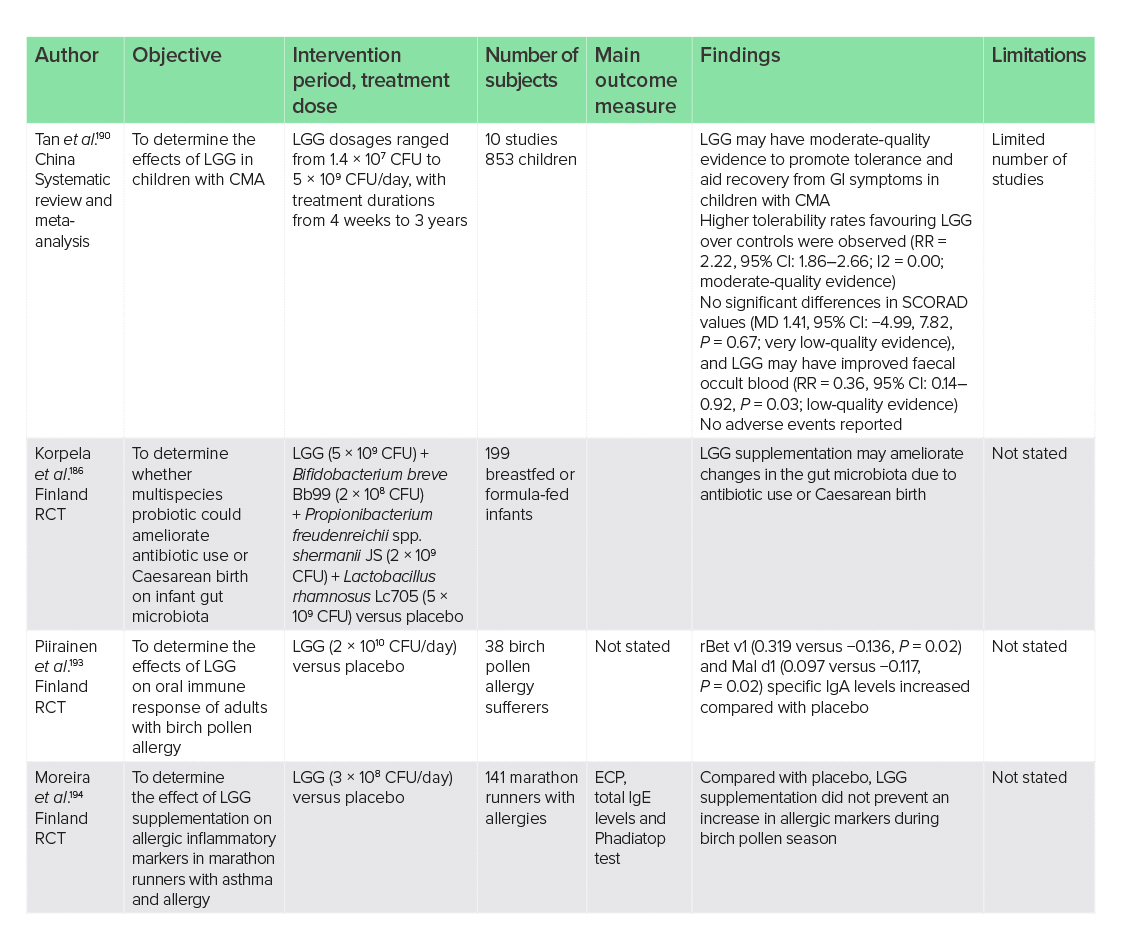
CFU, colony-forming units; CI, confidence interval; CMA, cow’s milk allergy; ECP, eosinophil cationic protein; GI, gastrointestinal; LGG, Lactobacillus rhamnosus GG; MD, mean difference; RCT, randomised-controlled trial; RR, relative risk; SCORAD, Scoring of Atopic Dermatitis.
Dermatitis and eczema
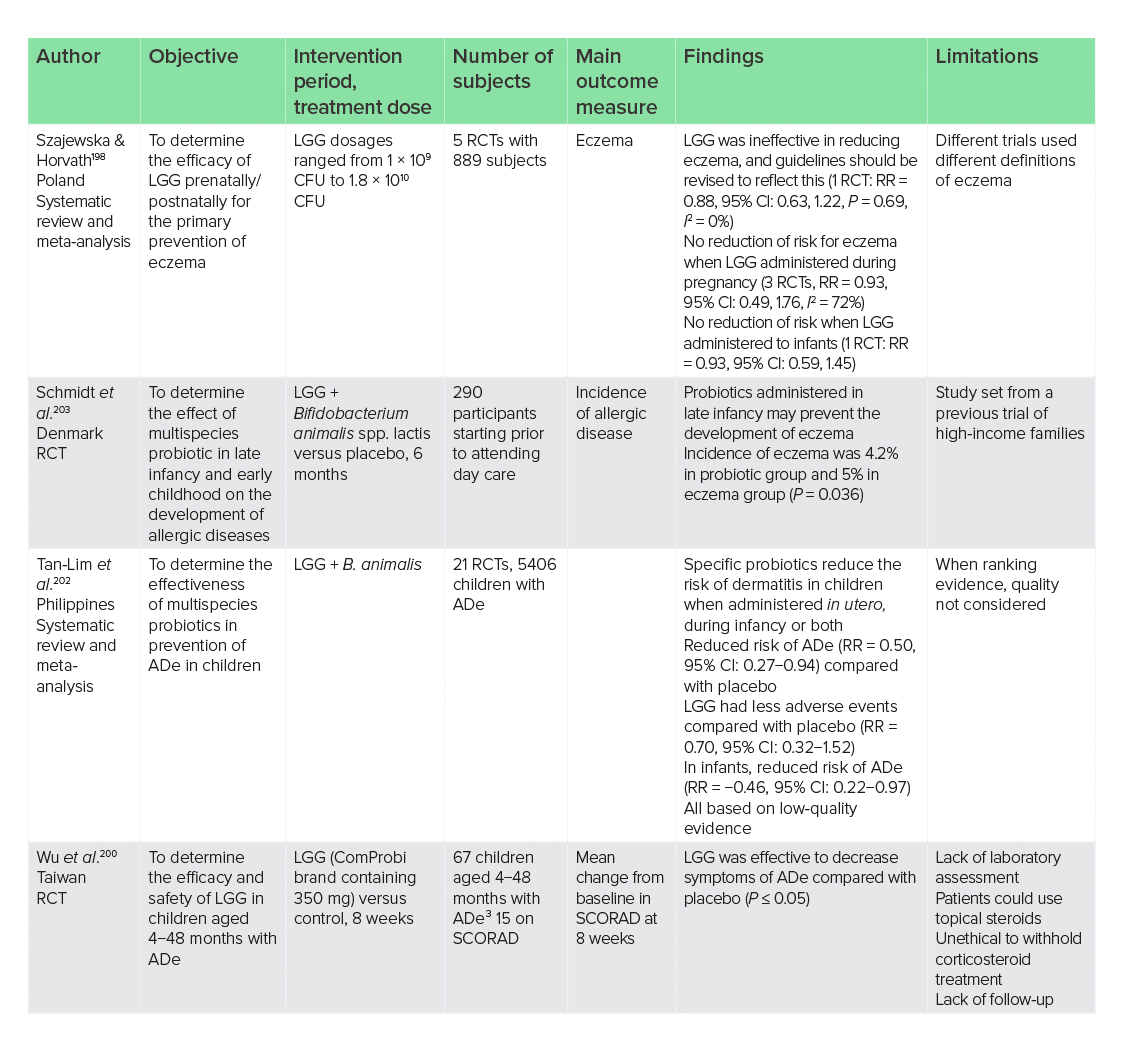
Ade, atopic dermatitis; CFU, colony-forming units; CI, confidence interval; LGG, Lactobacillus rhamnosus GG; RCT, randomised-controlled trial; RR, relative risk; SCORAD, Scoring of Atopic Dermatitis.
Wounds
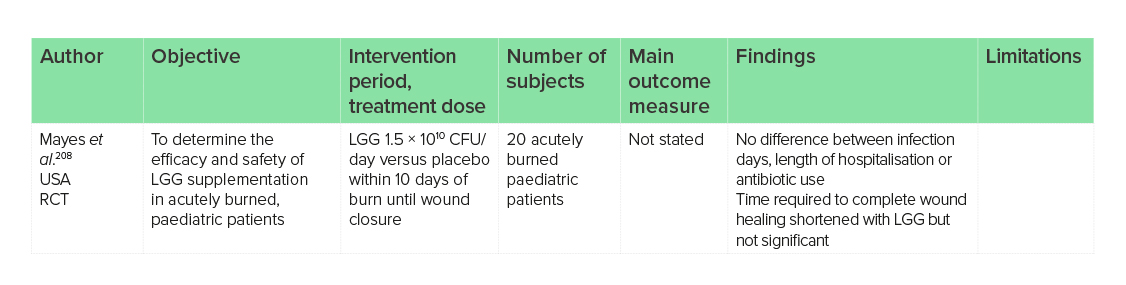
CFU, colony-forming units; LGG, Lactobacillus rhamnosus GG; RCT, randomised-controlled trial.
Dental caries
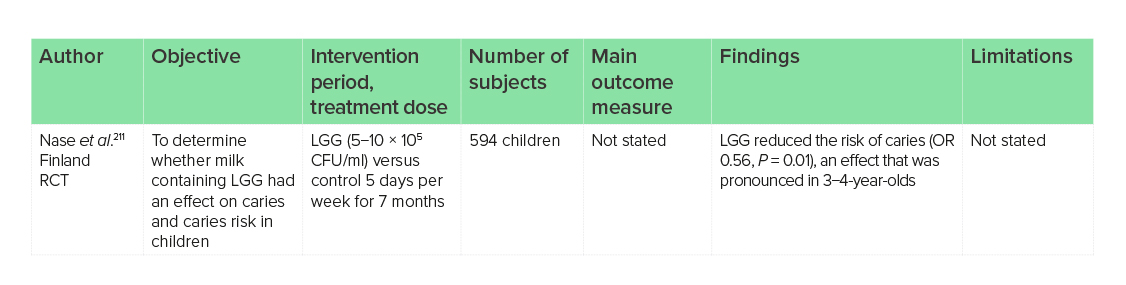
CFU, colony-forming units; LGG, Lactobacillus rhamnosus GG; OR, odds ratio; RCT, randomised-controlled trial.
Vaccine adjuvant
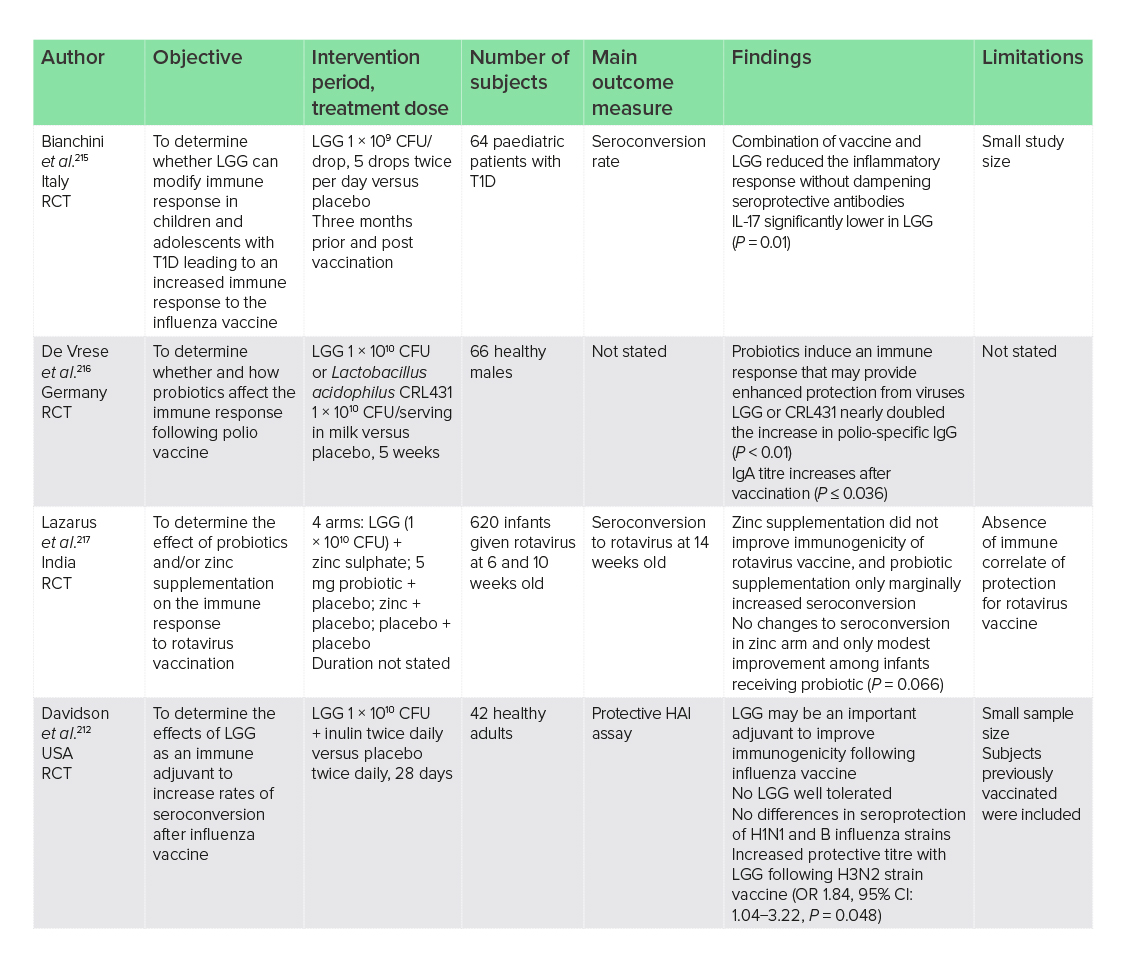
CFU, colony-forming units; CI, confidence interval; HAI, haemagglutinin inhibition; IL, interleukin; LGG, Lactobacillus rhamnosus GG; OR, odds ratio; RCT, randomised-controlled trial; T1D, type 1 diabetes.
Acknowledgements
Author contributions: C. Steele carried out the literature review and formulated the manuscript.
Peer-reviewers and editors: the Nutritional Medicine Institute thanks the peer-reviewers and editors for their important contributions.
Funding: Open Access publication was supported by an unrestricted donation from Pure Encapsulations, Sudbury, MA, USA. No other funding or sponsorship has been received for this work.
Declaration of interest: C. Steele has received consultancy fees from Pure Encapsulations, Sudbury, MA, USA. This article is the independent work of the author and Pure Encapsulations was not involved in the decision to publish this research.
References
1 Report F and AO of the UN and WHOEC (2001) Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Acid.
2 Capurso, L. (2019) Thirty Years of Lactobacillus rhamnosus GG: A review. J. Clin. Gastroenterol., 53 (Suppl 1), S1−S41. doi:10.1097/MCG.0000000000001170
3 Flesch, A. T., Tonial, S. T., Contu, P. D. C. & Damin, D. C. (2017) Perioperative synbiotics administration decreases postoperative infections in patients with colorectal cancer: a randomized, double-blind clinical trial. Rev. Col. Bras. Cir., 44 (6), 567−573. doi:10.1590/0100-69912017006004
4 Francavilla, R. et al. (2010) A randomized controlled trial of lactobacillus GG in children with functional abdominal pain. Pediatrics, 126 (6), e1445−e1452. doi:10.1542/peds.2010-0467
5 Du, X. et al. (2019) Efficacy of probiotic supplementary therapy for asthma, allergic rhinitis, and wheeze: A meta-analysis of randomized controlled trials. Allergy Asthma Proc., 40 (4), 250−260. doi:10.2500/aap.2019.40.4227
6 Rianda, D., Agustina, R., Setiawan, E. A. & Manikam, N. R. M. (2019) Effect of probiotic supplementation on cognitive function in children and adolescents: A systematic review of randomised trials. Benef. Microbes, 10 (8), 873−882. doi:10.3920/BM2019.0068
7 Tuomola, E. M., Ouwehand, A. C. & Salminen, S. J. (1999) The effect of probiotic bacteria on the adhesion of pathogens to human intestinal mucus. FEMS Immunol. Med. Microbiol., 26 (2), 137−142. doi:10.1016/S0928-8244(99)00131-5
8 Seth, A., Yan, F., Polk, D. & Rao, R. (2008) Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC- and MAP kinase-dependent mechanism. Am. J. Physiol. Gastrointest. Liver Physiol., 294 (4), G1060−G1069. doi:10.1152/ajpgi.00202.2007
9 Yan, F., Cao, H., Cover, T. L., Whitehead, R., Washington, M. K .& Polk, D. B. (2007) Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology, 132 (2), 562−575. doi:10.1053/j.gastro.2006.11.022
10 Claes, I. J. J. et al. (2012) Lipoteichoic acid is an important microbe-associated molecular pattern of Lactobacillus rhamnosus GG. Microb. Cell Fact., 11, 2−9. doi:10.1186/1475-2859-11-161
11 Sniffen, J. C., McFarland, L. V., Evans, C. T. & Goldstein, E. J. C. (2018) Choosing an appropriate probiotic product for your patient: An evidence-based practical guide. PLoS One, 13 (12), 1−22. doi:10.1371/journal.pone.0209205
12 Goldin, B. R., Gorbach, S. L., Saxelin, M., Barakat, S., Gualtieri, L. & Salminen, S. (1992) Survival of Lactobacillus species (strain GG) in human gastrointestinal tract. Dig. Dis. Sci., 37 (1), 121−128. doi:10.1007/BF01308354
13 Sepp, E., Mikelsaar, M. & Salminen, S. (1993) Effect of administration of lactobacillus casei strain GG on the gastrointestinal microbiota of newborns. Microb. Ecol. Health Dis., 6 (6), 309−314. doi:10.3109/08910609309141340
14 Kankainen, M. et al. (2009) Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human-mucus binding protein. Proc. Natl Acad. Sci. USA, 106 (40), 17 193−17 198. doi:10.1073/pnas.0908876106
15 Motherway, M. O. C. et al. (2011) Functional genome analysis of Bifidobacterium breve UCC2003 reveals type IVb tight adherence (Tad) pili as an essential and conserved host-colonization factor. Proc. Natl Acad. Sci. USA, 108 (27), 11 217−11 222. doi:10.1073/pnas.1105380108
16 Douillard, F. P. et al. (2013) Comparative genomic and functional analysis of 100 Lactobacillus rhamnosus strains and their comparison with strain GG. PLoS Genet., 9 (8), e1003683. doi:10.1371/journal.pgen.1003683
17 Nor, M. H. M. et al. (2021) The effect of probiotics (Mcp® bcmc® strains) on hepatic steatosis, small intestinal mucosal immune function, and intestinal barrier in patients with non-alcoholic fatty liver disease. Nutrients, 13 (9), 3192. doi:10.3390/nu13093192
18 Kwak, D. S. et al. (2014) Short-term probiotic therapy alleviates small intestinal bacterial overgrowth, but does not improve intestinal permeability in chronic liver disease. Eur. J. Gastroenterol. Hepatol., 26 (12), 1353−1359. doi:10.1097/MEG.0000000000000214
19 Lebeer, S. et al. (2012) Functional analysis of lactobacillus rhamnosus GG pili in relation to adhesion and immunomodulatory interactions with intestinal epithelial cells. Appl. Environ. Microbiol., 78 (1), 185−193. doi:10.1128/AEM.06192-11
20 Li, Y. et al. (2020) Inhibitory effects of the Lactobacillus rhamnosus GG effector protein HM0539 on inflammatory response through the TLR4/MyD88/NF-кB axis. Front. Immunol., 11 (October), 1−12. doi:10.3389/fimmu.2020.551449
21 Kuzmich, N. N., Sivak, K. V., Chubarev, V. N., Porozov, Y. B., Savateeva-Lyubimova, T. N. & Peri, F. (2017) TLR4 signaling pathway modulators as potential therapeutics in inflammation and sepsis. Vaccines, 5 (4), 1−25. doi:10.3390/vaccines5040034
22 Ciesielska, A., Matyjek, M. & Kwiatkowska, K. (2021) TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol. Life Sci., 78 (4), 1233−1261. doi:10.1007/s00018-020-03656-y
23 Roshan, M. H. K., Tambo, A. & Pace, N. P. (2016) The role of TLR2, TLR4, and TLR9 in the pathogenesis of atherosclerosis. Int. J. Inflam., 2016, 1532832. doi:10.1155/2016/1532832
24 Kamba, A., Lee, I.-.A. & Mizoguchi, E. (2013) Potential association between TLR4 and chitinase 3-like 1 (CHI3L1/YKL-40) signaling on colonic epithelial cells in inflammatory bowel disease and colitis-associated cancer. Curr. Mol. Med., 13 (7), 1110−1121. doi:10.2174/1566524011313070006
25 Wegh, C. A. M., Benninga, M. A. & Tabbers, M. M. (2018) Effectiveness of probiotics in children with functional abdominal pain disorders and functional constipation a systematic review. J. Clin. Gastroenterol., 52 (00), S10−S26. doi:10.1097/MCG.0000000000001054
26 Pedersen, N. et al. (2014) Ehealth: Low FODMAP diet vs Lactobacillus rhamnosus GG in irritable bowel syndrome. World J. Gastroenterol., 20 (43), 16 215−16 226. doi:10.3748/wjg.v20.i43.16215
27 Yoon, J. S. et al. (2014) Effect of multispecies probiotics on irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. J. Gastroenterol. Hepatol., 29 (1), 52−59. doi:10.1111/jgh.12322
28 Yan, F. et al. (2011) Colon-specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through an EGFR-dependent mechanism. J. Clin. Invest., 121 (6), 2242−2253. doi:10.1172/JCI44031
29 Hudault, S., Lievin, V., Bernet-Camard, M. & Servin, A. (1997) Antagonistic activity exerted in vitro and in vivo by Lactobacillus casei (strain GG) against Salmonella typhimurium C5 infection. Appl. Environ. Microbiol., 63 (2), 513−518.
30 Zhang, Y. et al. (2011) Antimicrobial activity against Shigella sonnei and probiotic properties of wild lactobacilli from fermented food. Microbiol. Res., 167 (1), 27−31. doi:10.1016/j.micres.2011.02.006
31 Meurman, J. H., Antila, H., Korhonen, A. & Salminen, S. (1995) Effect of Lactobacillus rhamnosus strain GG (ATCC 53103) on the growth of Streptococcus sobrinus in vitro. Eur. J. Oral Sci., 103 (4), 253−258. doi:10.1111/j.1600-0722.1995.tb00169.x
32 Silva, M., Jacobus, N. V., Deneke, C. & Gorbachl, S. L. (1987) Antimicrobial substance from a human Lactobacillus strain. Antimicrob. Agents Chemother.,
31 (8), 1231−1233.
33 Mattar, A., Drongowski, R., Coran, A. & Harmon, C. (2001) Effect of probiotics on enterocyte bacterial translocation in vitro. Pediatr. Surg. Int., 17 (4), 265−268. https://link-springer-com.ezproxy.unibo.it/content/pdf/10.1007%2Fs003830100591.pdf%0Ahttp://ovidsp.ovid.com/ovidweb.
34 Szachta, P., Ignyś, I. & Cichy, W. (2011) An evaluation of the ability of the probiotic strain Lactobacillus rhamnosus GG to eliminate the gastrointestinal carrier state of vancomycin-resistant enterococci in colonized children. J. Clin. Gastroenterol., 45 (10), 872−877. doi:10.1097/MCG.0b013e318227439f
35 De Keersmaecker, S. C. J., Verhoeven, T. L. A., Desair, J., Marchal, K., Vanderleyden, J. & Nagy, I. (2006) Strong antimicrobial activity of Lactobacillus rhamnosus GG against Salmonella typhimurium is due to accumulation of lactic acid. FEMS Microbiol. Lett., 259 (1), 89−96. doi:10.1111/j.1574-6968.2006.00250.x
36 Marianelli, C., Cifani, N. & Pasquali, P. (2010) Evaluation of antimicrobial activity of probiotic bacteria against Salmonella enterica subsp. enterica serovar typhimurium 1344 in a common medium under different environmental conditions. Res. Microbiol., 161 (8), 673−680. doi:10.1016/j.resmic.2010.06.007
37 Segers, M. E. & Lebeer, S. (2014) Towards a better understanding of Lactobacillus rhamnosus GG-host interactions. Microb. Cell. Fact., 13 (Suppl 1), S7. doi:10.1186/1475-2859-13-S1-S7
38 Tjalsma, H., Boleij, A., Marchesi, J. R. & Dutilh, B. E. (2012) A bacterial driver-passenger model for colorectal cancer: Beyond the usual suspects. Nat. Rev. Microbiol., 10 (8), 575−582. doi:10.1038/nrmicro2819
39 Wang, Y. H. et al. (2016) The efficacy and safety of probiotics for prevention of chemoradiotherapy-induced diarrhea in people with abdominal and pelvic cancer: A systematic review and meta-analysis. Eur. J. Clin. Nutr., 70 (11), 1246−1253. doi:10.1038/ejcn.2016.102
40 Rafter, J. et al. (2007) Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am. J. Clin. Nutr., 85 (2), 488−496. doi:10.1093/ajcn/85.2.488
41 Roller, M., Clune, Y., Collins, K., Rechkemmer, G. & Watzl, B. (2007) Consumption of prebiotic inulin enriched with oligofructose in combination with the probiotics Lactobacillus rhamnosus and Bifidobacterium lactis has minor effects on selected immune parameters in polypectomised and colon cancer patients. Br. J. Nutr., 97 (4), 676−684. doi:10.1017/S0007114507450292
42 Banna, G. L. et al. Anticancer oral therapy: Emerging related issues. Cancer Treat Rev., 36 (8), 595−605. doi:10.1016/j.ctrv.2010.04.005
43 Benson, A. B. et al. (2004) Recommended guidelines for the treatment of cancer treatment-induced diarrhea. J. Clin. Oncol., 22 (14), 2918−2926. doi:10.1200/JCO.2004.04.132
44 Österlund, P. et al. (2007) Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: A randomised study. Br. J. Cancer, 97 (8), 1028−1034. doi:10.1038/sj.bjc.6603990
45 Wroblewski, L. E., Peek, R. M. &Wilson, K. T. (2010) Helicobacter pylori and gastric cancer: Factors that modulate disease risk. Clin. Microbiol. Rev., 23 (4), 713−739. doi:10.1128/CMR.00011-10
46 Myllyluoma, E. et al. (2005) Probiotic supplementation improves tolerance to Helicobacter pylori eradication therapy − A placebo-controlled, double-blind randomized pilot study. Aliment. Pharmacol. Ther., 21 (10), 1263−1272. doi:10.1111/j.1365-2036.2005.02448.x
47 Armuzzi, A. et al. (2001) Effect of Lactobacillus GG supplementation on antibiotic-associated gastrointestinal side effects during Helicobacter pylori eradication therapy: A pilot study. Digestion, 63 (1), 1−7. doi:10.1159/000051865
48 Lages, P. C., Generoso, S. V. & Correia, M. I. T. D. (2018) Postoperative symbiotic in patients with head and neck cancer: A double-blind randomised trial. Br. J. Nutr., 119 (2), 190−195. doi:10.1017/S0007114517003403
49 Longstreth, G. F., Thompson, W. G., Chey, W. D., Houghton, L. A., Mearin, F. & Spiller, R. C. (2006) Functional bowel disorders. Gastroenterology, 130 (5), 1480−1491. doi:10.1053/j.gastro.2005.11.061
50 Sloan Pharma, US WorldMeds (2018) Zelnorm (tegaserod maleate): FDA Joint Meeting of the Gastrointestinal Drugs Advisory Committee Briefing Document. https://www.fda.gov/media/119013/download
51 El-Salhy, M. (2015) Recent advances in the diagnosis of irritable bowel syndrome. Expert Rev. Gastroenterol. Hepatol., 9 (9), 1161−1174. doi:10.1586/17474124.2015.1067138
52 Carroll, I. et al. (2011) Molecular analysis of the luminal- and mucosal-associated intestinal microbiota in diarrhea-predominant irritable bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol., 301 (5), G799–G807.
53 Malinen, E. et al. (2005) Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am. J. Gastroenterol., 100 (2), 373−382. doi:10.1111/j.1572-0241.2005.40312.x
54 Lyra, A. et al. (2010) Effect of a multispecies probiotic supplement on quantity of irritable bowel syndrome-related intestinal microbial phylotypes. BMC Gastroenterol., 10, 110. doi:10.1186/1471-230X-10-110
55 Canakis, A., Haroon, M. & Weber, H. C. (2020) Irritable bowel syndrome and gut microbiota. Curr. Opin. Endocrinol. Diabetes Obes., 27 (1), 28−35. doi:10.1097/MED.0000000000000523
56 Mättö, J. et al. (2005) Composition and temporal stability of gastrointestinal microbiota in irritable bowel syndrome − A longitudinal study in IBS and control subjects. FEMS Immunol. Med. Microbiol., 43 (2), 213−222. doi:10.1016/j.femsim.2004.08.009
57 Rutten, J. M. T. M., Korterink, J. J., Venmans, L. M. A. J., Benninga, M. A. & Tabbers, M. M. (2015) Nonpharmacologic treatment of functional abdominal pain disorders: A systematic review. Pediatrics, 135 (3), 522−535. doi:10.1542/peds.2014-2123
58 Horvath, A., Dziechciarz, P. & Szajewska, H. (2011) Meta-analysis: Lactobacillus rhamnosus GG for abdominal pain-related functional gastrointestinal disorders in childhood. Aliment. Pharmacol. Ther., 33 (12), 1302−1310. doi:10.1111/j.1365-2036.2011.04665.x
59 Gawrońska, A., Dziechciarz, P., Horvath, A. & Szajewska, H. (2007) A randomized double-blind placebo-controlled trial of Lactobacillus GG for abdominal pain disorders in children. Aliment. Pharmacol. Ther., 25 (2), 177−184. doi:10.1111/j.1365-2036.2006.03175.x
60 Walker, C. et al. (2013) Global burden of childhood pneumonia and diarrhoea. Lancet, 381, 1405−1416.
61 Schnadower, D. et al. (2018) Lactobacillus rhamnosus GG versus placebo for acute gastroenteritis in children. N. Engl. J. Med., 379 (21), 2002−2014. doi:10.1056/nejmoa1802598
62 Surawicz, C. M. (2003) Probiotics, antibiotics-associated diarrhoea and Clostridium diffucile diarrhoea in humans. Bailliere’s Best Pract. Res. Clin. Gastroenterol., 17 (5), 775−783. doi:10.1016/S1521-6918(03)00054-4
63 Tytgat, H. L. P. et al. (2016) Lactobacillus rhamnosus GG outcompetes Enterococcus faecium via mucus-binding pili: Evidence for a novel and heterospecific probiotic mechanism. Appl. Environ. Microbiol., 82 (19), 5756−5762. doi:10.1007/s40278-016-15938-2
64 Ding, Y. H. et al. (2017) The regulation of immune cells by Lactobacilli: A potential therapeutic target for anti-atherosclerosis therapy. Oncotarget, 8 (35), 59 915−59 928. doi:10.18632/oncotarget.18346
65 Korpela, K., Salonen, A., Virta, L. J., Kumpu, M., Kekkonen, R. A. & De Vos, W. M. (2016) Lactobacillus rhamnosus GG intake modifies preschool children’s intestinal microbiota, alleviates penicillin-associated changes, and reduces antibiotic use. PLoS One, 11 (4), 1−16. doi:10.1371/journal.pone.0154012
66 Szajewska, H. & Kołodziej, M. (2015) Systematic review with meta-analysis: Lactobacillus rhamnosus GG in the prevention of antibiotic-associated diarrhoea in children and adults. Aliment. Pharmacol. Ther., 42 (10), 1149−1157. doi:10.1111/apt.13404
67 Agamennone, V., Krul, C. A. M., Rijkers, G. & Kort, R. (2019) A practical guide for probiotics applied to the case of antibiotic-associated diarrhea in the Netherlands. Pharm. Weekbl., 154 (6), 17−24.
68 Makras, L. et al. (2006) Kinetic analysis of the antibacterial activity of probiotic lactobacilli towards Salmonella enterica serovar Typhimurium reveals a role for lactic acid and other inhibitory compounds. Res. Microbiol., 157 (3), 241−247. doi:10.1016/j.resmic.2005.09.002
69 Szajewska, H. et al. (2016) Probiotics for the prevention of antibiotic-associated diarrhea in children. J. Pediatr. Gastroenterol. Nutr., 62 (3), 495−506. doi:10.1097/MPG.0000000000001081
70 Li, Y. T. et al. (2019) Efficacy of Lactobacillus rhamnosus GG in treatment of acute pediatric diarrhea: A systematic review with meta-analysis. World J. Gastroenterol., 25 (33), 4999−5016. doi:10.3748/wjg.v25.i33.4999
71 Fang, S.-B., Lee, H. C., Hu, J. J., Hou, S. Y., Liu, H. L. & Fang, H. W. (2009) Dose-dependent effect of Lactobacillus rhamnosus on quantitative reduction of faecal rotavirus shedding in children. J. Trop. Pediatr., 55 (5), 297−301. doi:10.1093/tropej/fmp001
72 Sindhu, K. N. C. et al. (2014) Immune response and intestinal permeability in children with acute gastroenteritis treated with Lactobacillus rhamnosus GG: A randomized, double-blind, placebo-controlled trial. Clin. Infect. Dis., 58 (8), 1107−1115. doi:10.1093/cid/ciu065
73 Zhang, Y. Z. & Li, Y. Y. (2014) Inflammatory bowel disease: Pathogenesis. World J. Gastroenterol., 20 (1), 91−99. doi:10.3748/wjg.v20.i1.91
74 Danese, S. & Fiocchi, C. (2006) Etiopathogenesis of inflammatory bowel diseases. World J. Gastroenterol., 12 (30), 4807−4812. doi:10.3748/wjg.v12.i30.4807
75 Kugathasan, S. & Fiocchi, C. (2007) Progress in basic inflammatory bowel disease research. Semin. Pediatr. Surg., 16 (3), 146−153. doi:10.1053/j.sempedsurg.2007.04.002
76 Podolsky, D. (2002) Inflammatory bowel disease. Prim. Care Manag. Community-Acquired Pneumonia, 347 (6), 417−419. doi:10.2217/EBO.11.37
77 Martinez-Medina, M., Aldeguer, X., Gonzalez-Huix, F., Acero, D. & Garcia-Gil, L. J. (2006) Abnormal microbiota composition in the ileocolonic mucosa of Crohn’s disease patients as revealed by polymerase chain reaction-denaturing gradient gel electrophoresis. Inflamm. Bowel Dis., 12 (12), 1136−1145. doi:10.1097/01.mib.0000235828.09305.0c
78 Frank, D. N., St Amand, A. L., Feldman, R. A., Boedeker, E. C., Harpaz, N. & Pace, N. R. (2007) Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl Acad. Sci. USA, 104 (34), 13 780−13 785. doi:10.1073/pnas.0706625104
79 Jonkers, D., Penders, J., Masclee, A. & Pierik, M. (2012) Probiotics in the management of inflammatory bowel disease. Drugs, 72 (6), 803−823. doi:10.2165/11632710-000000000-00000
80 Shen, J., Ran, H. Z., Yin, M. H., Zhou, T. X. & Xiao, D. S. (2009) Meta-analysis: The effect and adverse events of Lactobacilli versus placebo in maintenance therapy for Crohn disease. Intern. Med. J., 39 (2), 103−109. doi:10.1111/j.1445-5994.2008.01791.x
81 Bousvaros, A. et al. (2005) A randomized, double-blind trial of lactobacillus GG versus placebo in addition to standard maintenance therapy for children with Crohn’s disease. Inflamm. Bowel Dis., 11 (9), 833−839. doi:10.1097/01.MIB.0000175905.00212.2c
82 Lorea Baroja, M., Kirjavainen, P. V., Hekmat, S. & Reid, G. (2007) Anti-inflammatory effects of probiotic yogurt in inflammatory bowel disease patients. Clin. Exp. Immunol., 149 (3), 470−479. doi:10.1111/j.1365-2249.2007.03434.x
83 Zuo, T. & Ng, S. C. (2018) The gut microbiota in the pathogenesis and therapeutics of inflammatory bowel disease. Front. Microbiol., 9, 2247. doi:10.3389/fmicb.2018.02247
84 Patterson, E. E. et al. (2016) Gut microbiota, obesity and diabetes. Postgrad. Med. J., 92 (1087), 286−300. doi:10.1136/postgradmedj-2015-133285
85 Forssten, S. D., Korczyńska, M. Z., Zwijsen, R. M. L., Noordman, W. H., Madetoja, M. & Ouwehand, A. C. (2013) Changes in satiety hormone concentrations and feed intake in rats in response to lactic acid bacteria. Appetite, 71, 16−21. doi:10.1016/j.appet.2013.06.093
86 Riva, A. et al. (2017) Pediatric obesity is associated with an altered gut microbiota and discordant shifts in Firmicutes populations. Environ. Microbiol., 19 (1), 95−105. doi:10.1111/1462-2920.13463
87 Bervoets, L. et al. (2013) Differences in gut microbiota composition between obese and lean children: A cross-sectional study. Gut Pathog., 5 (1), 1−10. doi:10.1186/1757-4749-5-10
88 Munukka, E. et al. (2012) Women with and without metabolic disorder differ in their gut microbiota composition. Obesity, 20 (5), 1082−1087. doi:10.1038/oby.2012.8
89 Ley, R., Turnbaugh, P., Klein, S. & Gordon, J. (2006) Human gut microbes associated with obesity. Adv. Astronaut Sci., 444, 41−54. doi:10.1038/nature4441021a
90 Okesene-Gafa, K. A. M. et al. (2019) Effect of antenatal dietary interventions in maternal obesity on pregnancy weight-gain and birthweight: Healthy Mums and Babies (HUMBA) randomized trial. Am. J. Obstet. Gynecol., 221 (2), 152.e1−152.e13. doi:10.1016/j.ajog.2019.03.003
91 Callaway, L. K. et al. (2019) Probiotics for the prevention of gestational diabetes mellitus in overweight and obese women: Findings from the SPRING double-blind randomized controlled trial. Diabetes Care, 42 (3), 364−371. doi:10.2337/dc18-2248
92 Kekkonen, R. A. et al. (2008) Effect of probiotic Lactobacillus rhamnosus GG intervention on global serum lipidomics profiles in healthy adults. World J. Gastroenterol., 14 (20), 3188−3194. doi:10.3748/wjg.14.3188
93 van der Veen, J. N., Kennelly, J. P., Wan, S., Vance, J. E., Vance, D. E. & Jacobs, R. L. (2017) The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta Biomembr., 1859 (9), 1558−1572. doi:10.1016/j.bbamem.2017.04.006
94 Yu, Z., Peng, Q. & Huang, Y. (2019) Potential therapeutic targets for atherosclerosis in sphingolipid metabolism. Clin. Sci., 133 (6), 763−776. doi:10.1042/CS20180911
95 Hu, H. et al. (2020) Intestinal microbiome and NAFLD: molecular insights and therapeutic perspectives. J. Gastroenterol., 55 (2), 142−158. doi:10.1007/s00535-019-01649-8
96 Cobbina, E. & Akhlaghi, F. (2017) Non-alcoholic fatty liver disease (NAFLD) − pathogenesis, classification, and effect on drug metabolizing enzymes and transporters. Drug Metab. Rev., 49 (2), 197−211. doi:10.1080/03602532.2017.1293683
97 Tarao, K., So, K., Moroi, T., Ikeuchi, T. & Suyama, T. (1977) Detection of endotoxin in plasma and ascitic fluid of patients with cirrhosis: its clinical significance. Gastroenterology, 73 (3), 539−542. doi:10.1016/s0016-5085(19)32137-7
98 Pinzone, M. R., Celesia, B. M., Di Rosa, M., Cacopardo, B. & Nunnari, G. (2012) Microbial translocation in chronic liver diseases. Int. J. Microbiol., 2012, 694629. doi:10.1155/2012/694629
99 Vajro, P. et al. (2011) Effects of Lactobacillus rhamnosus strain GG in pediatric obesity-related liver disease. J. Pediatr. Gastroenterol. Nutr., 52 (6), 740−743. doi:10.1097/MPG.0b013e31821f9b85
100 Schwab, J. H. (1993) Phlogistic properties of peptidoglycan-polysaccharide polymers from cell walls of pathogenic and normal-flora bacteria which colonize humans. Infect. Immun., 61 (11), 4535−4539. doi:10.1128/iai.61.11.4535-4539.1993
101 Larsen, N. et al. (2010) Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One, 5 (2), e9085. doi:10.1371/journal.pone.0009085
102 Sato, J. et al. (2014) Gut dysbiosis and detection of “Live gut bacteria” in blood of Japanese patients with type 2 diabetes. Diabetes Care, 37 (8), 2343−2350. doi:10.2337/dc13-2817
103 Asemi, Z., Zare, Z., Shakeri, H., Sabihi, S. S. & Esmaillzadeh, A. (2013) Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann. Nutr. Metab., 63 (1-2), 1−9. doi:10.1159/000349922
104 Tabuchi, M. et al. (2003) Antidiabetic effect of lactobacillus gg in streptozotocin-induced diabetic rats. Biosci. Biotechnol. Biochem., 67 (6), 1421−1424. doi:10.1271/bbb.67.1421
105 Sanborn, V. E., Azcarate-Peril, M. A. & Gunstad, J. (2020) Lactobacillus rhamnosus GG and HbA1c in middle age and older adults without type 2 diabetes mellitus: A preliminary randomized study. Diabetes Metab. Syndr. Clin. Res. Rev., 14 (5), 907−909. doi:10.1016/j.dsx.2020.05.034
106 Laitinen, K., Poussa, T. & Isolauri, E. (2009) Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: A randomised controlled trial. Br. J. Nutr., 101 (11), 1679−1687. doi:10.1017/S0007114508111461
107 Werlin, S. L. et al. (2010) Evidence of intestinal inflammation in patients with cystic fibrosis. J. Pediatr. Gastroenterol. Nutr., 51 (3), 304−308. doi:10.1097/MPG.0b013e3181d1b013
108 Bruzzese, E. et al. (2004) Intestinal inflammation is a frequent feature of cystic fibrosis and is reduced by probiotic administration. Aliment. Pharmacol. Ther., 20 (7), 813−819. doi:10.1111/j.1365-2036.2004.02174.x
109 Neri, L. D. C. L., Taminato, M. & Da Silva Filho, L. V. R. F. (2019) Systematic review of probiotics for cystic fibrosis patients: Moving forward. J. Pediatr. Gastroenterol. Nutr., 68 (3), 394−399. doi:10.1097/MPG.0000000000002185
110 Bruzzese, E. et al. (2014) Disrupted intestinal microbiota and intestinal inflammation in children with cystic fibrosis and its restoration with lactobacillus gg: A randomised clinical trial. PLoS One, 9 (2), 1−12. doi:10.1371/journal.pone.0087796
111 Bruzzese, E. et al. (2007) Effect of Lactobacillus GG supplementation on pulmonary exacerbations in patients with cystic fibrosis: A pilot study. Clin. Nutr., 26 (3), 322−328. doi:10.1016/j.clnu.2007.01.004
112 Bruzzese, E. et al. (2018) Lack of efficacy of Lactobacillus GG in reducing pulmonary exacerbations and hospital admissions in children with cystic fibrosis: A randomised placebo controlled trial. J. Cyst. Fibros., 17 (3), 375−382. doi:10.1016/j.jcf.2017.10.014
113 Byars, S., Stearns, S. & Boomsma, J. (2018) Association of long-term risk of respiratory, allergic, and infectious diseases with removal of adenoids and tonsils in childhood. JAMA Otolaryngol. Neck Surg., 144 (7), 594−603.
114 Swanljung, E. et al. (2015) Lactobacillus rhamnosus GG in adenoid tissue: Double-blind, placebo-controlled, randomized clinical trial. Acta Otolaryngol., 135 (8), 824−830. doi:10.3109/00016489.2015.1027412
115 Tapiovaara, L. et al. (2016) Human rhinovirus in experimental infection after peroral Lactobacillus rhamnosus GG consumption, a pilot study. Int. Forum Allergy Rhinol., 6 (8), 848−853. doi:10.1002/alr.21748
116 Kumpu, M. et al. (2015) Effect of live and inactivated Lactobacillus rhamnosus GG on experimentally induced rhinovirus colds: Randomised, double blind, placebo-controlled pilot trial. Benef. Microbes, 6 (5), 631−639. doi:10.3920/BM2014.0164
117 Smith, T. J., Rigassio-Radler, D., Denmark, R., Haley, T. & Touger-Decker, R. (2013) Effect of Lactobacillus rhamnosus LGG® and Bifidobacterium animalis ssp. lactis BB-12® on health-related quality of life in college students affected by upper respiratory infections. Br. J. Nutr., 109 (11), 1999−2007. doi:10.1017/S0007114512004138
118 Alter, S. J., Bennett, J. S., Koranyi, K., Kreppel, A. & Simon, R. (2015) Common childhood viral infections. Curr. Probl. Pediatr. Adolesc. Health Care, 45 (2), 21−53. doi:10.1016/j.cppeds.2014.12.001
119 Koefoed, B., Nielsen, A. & Keiding, L. (2002) The impact of selected environmental factors on the morbidity of children in day care centres. Ugeskl Laeger., 164 (49), 5759−5764.
120 M’Rabet, L., Vos, A. P., Boehm, G. & Garssen, J. (2008) Breast-feeding and its role in early development of the immune system in infants: Consequences for health later in life. J. Nutr., 138 (9), 1782−1790. doi:10.1093/jn/138.9.1782s
121 Laursen, R. P., Larnkjær, A., Ritz, C., Hauger, H., Michaelsen, K. F. & Mølgaard, C. (2017) Probiotics and child care absence due to infections: A randomized controlled trial. Pediatrics, 140 (2), e20170735. doi:10.1542/peds.2017-0735
122 Laursen, R. P. & Hojsak, I. (2018) Probiotics for respiratory tract infections in children attending day care centers—a systematic review. Eur. J. Pediatr., 177 (7), 979−994. doi:10.1007/s00431-018-3167-1
123 Liu, S., Hu, P., Du, X., Zhou, T. & Pei, X. (2013) Lactobacillus rhamnosus GG supplementation for preventing respiratory infections in children: a meta-analysis of randomized, placebo-controlled trials. Indian Pediatr., 50 (16), 377−381. doi:10.2165/00128415-201113790-00083
124 Luoto, R., Ruuskanen, O., Waris, M., Kalliomaki, M., Salminen, S. & Isolauri, E. (2014) Prebiotic and probiotic supplementation prevents rhinovirus infections in preterm infants: A randomized, placebo-controlled trial. J. Allergy Clin. Immunol., 133, 405−413.
125 Hojsak, I., Abdović, S., Szajewska, H., Milošević, M., Krznarić, Ž. & Kolaček, S. (2010) Lactobacillus GG in the prevention of nosocomial gastrointestinal and respiratory tract infections. Pediatrics, 125 (5), e1171−e1177. doi:10.1542/peds.2009-2568
126 Harmes, K. M., Blackwood, R. A., Burrows, H. L., Cooke, J. M., Van Harrison, R. & Passamani, P. P. (2013) Otitis media: Diagnosis and treatment. Am. Fam. Physician, 88 (7), 435−440.
127 Tapiovaara, L. et al. (2014) Lactobacillus rhamnosus GG in the middle ear after randomized, double-blind, placebo-controlled oral administration. Int. J. Pediatr. Otorhinolaryngol., 78 (10), 1637−1641. doi:10.1016/j.ijporl.2014.07.011
128 Hatakka, K. et al. (2007) Treatment of acute otitis media with probiotics in otitis-prone children − A double-blind, placebo-controlled randomised study. Clin. Nutr., 26 (3), 314−321. doi:10.1016/j.clnu.2007.01.003
129 Lehtoranta, L. et al. (2012) Human bocavirus in the nasopharynx of otitis-prone children. Int. J. Pediatr. Otorhinolaryngol., 76 (2), 206−211. doi:10.1016/j.ijporl.2011.10.025
130 Walter, S. A. et al. (2013) Abdominal pain is associated with anxiety and depression scores in a sample of the general adult population with no signs of organic gastrointestinal disease. Neurogastroenterol. Motil., 25 (9), 741−e576. doi:10.1111/nmo.12155
131 Wang, H. X. & Wang, Y. P. (2016) Gut microbiota-brain axis. Chin. Med. J. (Engl.), 129 (19), 2373−2380. doi:10.4103/0366-6999.190667
132 Wu, Q. & Shah, N. P. (2017) High γ-aminobutyric acid production from lactic acid bacteria: Emphasis on Lactobacillus brevis as a functional dairy starter. Crit. Rev. Food Sci. Nutr., 57 (17), 3661−3672. doi:10.1080/10408398.2016.1147418
133 Dinan, T. G. & Cryan, J. F. (2017) The microbiome-gut-brain axis in health and disease. Gastroenterol. Clin. North Am., 46 (1), 77−89. doi:10.1016/j.gtc.2016.09.007
134 De Lorenzo, A. et al. (2017) Can psychobiotics intake modulate psychological profile and body composition of women affected by normal weight obese syndrome and obesity? A double blind randomized clinical trial. J. Transl. Med., 15 (1), 1−12. doi:10.1186/s12967-017-1236-2
135 Mohammadi, A. A. et al. (2016) The effects of probiotics on mental health and hypothalamic–pituitary–adrenal axis: A randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutr. Neurosci., 19 (9), 387−395. doi:10.1179/1476830515Y.0000000023
136 Moludi, J., Alizadeh, M., Mohammadzad, M. H. S. & Davari, M. (2019) The effect of probiotic supplementation on depressive symptoms and quality of life in patients after myocardial infarction: results of a preliminary double-blind clinical trial. Psychosom. Med., 81 (9), 770−777. doi:10.1097/PSY.0000000000000749
137 Chen, M. H. et al. (2018) Rapid inflammation modulation and antidepressant efficacy of a low-dose ketamine infusion in treatment-resistant depression: A randomized, double-blind control study. Psychiatry Res., 269, 207−211. doi:10.1016/j.psychres.2018.08.078
138 Traylor, C.S., Johnson, J. D., Kimmel, M. C. & Manuck, T. A. (2020) Effects of psychological stress on adverse pregnancy outcomes and nonpharmacologic approaches for reduction: an expert review. Am. J. Obstet. Gynecol. MFM, 2 (4), 100 229. doi:10.1016/j.ajogmf.2020.100229
139 Molyneaux, E., Poston, L., Ashurst-Williams, S. & Howard, L. M. (2014) Obesity and mental disorders during pregnancy and postpartum: A systematic review and meta-analysis. Obstet. Gynecol., 123 (4), 857−867. doi:10.1097/AOG.0000000000000170
140 Nagl, M., Linde, K., Stepan, H. & Kersting, A. (2015) Obesity and anxiety during pregnancy and postpartum: A systematic review. J. Affect. Disord., 186, 293−305. doi:10.1016/j.jad.2015.06.054
141 Steinig, J., Nagl, M., Linde, K., Zietlow, G. & Kersting, A. (2017) Antenatal and postnatal depression in women with obesity: a systematic review. Arch. Womens Ment. Health, 20 (4), 569−585. doi:10.1007/s00737-017-0739-4
142 Sheyholislami, H. & Connor, K. L. (2021) Are probiotics and prebiotics safe for use during pregnancy and lactation? A systematic review and meta-analysis. Nutrients, 13 (7), 2382. doi:10.3390/nu13072382
143 Dawe, J. P., McCowan, L. M. E., Wilson, J., Okesene-Gafa, K. A. M. & Serlachius, A. S. (2020) Probiotics and maternal mental health: a randomised controlled trial among pregnant women with obesity. Sci. Rep., 10 (1), 1−11. doi:10.1038/s41598-020-58129-w
144 Thapar, A. & Cooper, M. (2016) Attention deficit hyperactivity disorder. Lancet, 387 (10 024), 1240−1250. doi:10.1016/S0140-6736(15)00238-X
145 Mirkovic, B. & Gérardin, P. (2019) Asperger’s syndrome: What to consider? Encephale, 45 (2), 169−174. doi:10.1016/j.encep.2018.11.005
146 Bravo, J. A. et al. (2011) Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl Acad. Sci. USA, 108 (38), 16 050−16 055. doi:10.1073/pnas.1102999108
147 Enticott, P. G., Rinehart, N. J., Tonge, B. J., Bradshaw, J. L. & Fitzgerald, P. B. (2010) A preliminary transcranial magnetic stimulation study of cortical inhibition and excitability in high-functioning autism and Asperger disorder. Dev. Med. Child. Neurol., 52 (8), 179−183. doi:10.1111/j.1469-8749.2010.03665.x
148 Gareau, M. G. et al. (2011) Bacterial infection causes stress-induced memory dysfunction in mice. Gut, 60 (3), 307−317. doi:10.1136/gut.2009.202515
149 Pärtty, A., Kalliomäki, M., Wacklin, P., Salminen, S. & Isolauri, E. (2015) A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: A randomized trial. Pediatr. Res., 77 (6), 823−828. doi:10.1038/pr.2015.51
150 Cai, T. & Bartoletti, R. (2017) Asymptomatic bacteriuria in recurrent UTI − to treat or not to treat. GMS Infect. Dis., 5, Doc09. doi:10.3205/id000035
151 Echols, R. M., Tosiello, R. L., Haverstock, D. C. & Tice, A. D. (1999) Demographic, clinical, and treatment parameters influencing the outcome of acute cystitis. Clin. Infect. Dis., 29 (1), 113−119. doi:10.1086/520138
152 Ng, Q. X., Peters, C., Venkatanarayanan, N., Goh, Y. Y., Ho, C. Y. X. & Yeo, W. S. (2018) Use of Lactobacillus spp. to prevent recurrent urinary tract infections in females. Med. Hypotheses, 114, 49−54. doi:10.1016/j.mehy.2018.03.001
153 Zhang, H. et al. (2020) The postbiotic HM0539 from Lactobacillus rhamnosus GG prevents intestinal infection by enterohemorrhagic E. coli O157: H7 in mice. Nan Fang Yi Ke Da Xue Xue Bao, 40 (2), 211−218. doi:10.12122/j.issn.1673-4254.2020.02.12
154 Colodner, R., Edelstein, H., Chazan, B. & Raz, R. (2003) Vaginal colonization by orally administered Lactobacillus rhamnosus GG. Isr. Med. Assoc. J., 5 (11), 767−769.
155 Kontiokari, T., Sundqvist, K., Nuutinen, M., Pokka, T., Koskela, M. & Uhari, M. (2001) Randomised trial of cranberry-lingonberry juice and Lactobacilus GG drink for the prevention of urinary tract infections in women. Br. Med. J., 322 (7302), 1571.
156 Sadeghi-Bojd, S., Naghshizadian, R., Mazaheri, M., Ghane Sharbaf, F. & Assadi, F. (2020) Efficacy of probiotic prophylaxis after the first febrile urinary tract infection in children with normal urinary tracts. J. Pediatric Infect. Dis. Soc., 9 (3), 305−310. doi:10.1093/JPIDS/PIZ025
157 Waites, K., Canupp, K. & DeVivo, M. (1993) Epidemiology and risk factors for urinary tract infection in patients with spinal cord injury. Arch. Phys. Med. Rehabil., 74 (7), 691−695. doi:10.1016/S0022-5347(05)67157-1
158 Cardenas, D. D. & Hooton, T. M. (1995) Urinary tract infection in persons with spinal cord injury. Arch. Phys. Med. Rehabil., 76 (3), 272−280. doi:10.1016/S0003-9993(95)80615-6
159 Toh, S. L. et al. (2019) Probiotics [LGG-BB12 or RC14-GR1] versus placebo as prophylaxis for urinary tract infection in persons with spinal cord injury [ProSCIUTTU]: a randomised controlled trial. Spinal Cord, 57 (7), 550−561. doi:10.1038/s41393-019-0251-y
160 Tractenberg, R. E. et al. (2021) Effects of intravesical Lactobacillus rhamnosus GG on urinary symptom burden in people with neurogenic lower urinary tract dysfunction. PM R, 13 (7), 695−706. doi:10.1002/pmrj.12470
161 Yang, R. et al. (2019) Dynamic signatures of gut microbiota and influences of delivery and feeding modes during the first 6 months of life. Physiol. Genomics, 51 (8), 368−378.
162 Stewart, C. J. et al. (2018) Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature, 562 (7728), 583−588. doi:10.1038/s41586-018-0617-x
163 Kincaid, H. J., Nagpal, R. & Yadav, H. (2020) Microbiome-immune-metabolic axis in the epidemic of childhood obesity: Evidence and opportunities. Obes. Rev., 21 (2), 1−25. doi:10.1111/obr.12963
164 Slabuszewska-Jozwiak, A., Krzysztof Szymanski, J., Ciebiera, M., Sarecka-Hujar, B. & Jakiel, G. (2020) Pediatrics consequences of Caesarean section — a systematic review and meta-analysis. Int. J. Environ. Res. Public Health, 17, 8031.
165 Azad, M. et al. (2013) Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ, 185 (5), 385−394. doi:10.1503/cmaj.130147
166 Parnanen, K. et al. (2022) Early-life formula feeding is associated with infant gut microbiota alterations and an increased antibiotic resistance load. Am. J. Clin. Nutr., 115 (2), 407−421.
167 Chrzanowska-Liszewska, D., Seliga-Siwecka, J. & Kornacka, M. K. (2012) The effect of Lactobacillus rhamnosus GG supplemented enteral feeding on the microbiotic flora of preterm infants − double blinded randomized control trial. Early Hum. Dev., 88 (1), 57−60. doi:10.1016/j.earlhumdev.2011.07.002
168 Mantaring, J. et al. (2018) Effect of maternal supplement beverage with and without probiotics during pregnancy and lactation on maternal and infant health: A randomized controlled trial in the Philippines. BMC Pregnancy Childbirth, 18 (1), 1−12. doi:10.1186/s12884-018-1828-8
169 Vendt, N. et al. (2006) Growth during the first 6 months of life in infants using formula enriched with Lactobacillus rhamnosus GG: double-blind, randomized trial. J. Hum. Nutr. Diet, 19, 51−58.
170 Berglund, S. K. et al. (2016) Maternal, fetal and perinatal alterations associated with obesity, overweight and gestational diabetes: An observational cohort study (PREOBE). BMC Public Health, 16 (1), 1−12. doi:10.1186/s12889-016-2809-3
171 Luoto, R., Laitinen, K., Nermes, M. & Isolauri, E. (2010) Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: A double-blind, placebo-controlled study. Br. J. Nutr., 103 (12), 1792−1799. doi:10.1017/S0007114509993898
172 Pärtty, A., Lehtonen, L., Kalliomäki, M., Salminen, S. & Isolauri, E. (2015) Probiotic Lactobacillus rhamnosus GG therapy and microbiological programming in infantile colic: A randomized, controlled trial. Pediatr. Res., 78 (4), 470−475. doi:10.1038/pr.2015.127
173 Savino, F., Montanari, P., Galliano, I., Dapra, V. & Bergallo, M. (2020) Lactobacillus rhamnosus GG (ATCC 53103) for the management of infantile colic: a randomized controlled trial. Nutrients, 12, 1693.
174 Cabana, M., McKean, M., Beck, A. & Flaherman, V. (2019) A pilot analysis of early Lactobacillus rhamnosus GG for infant collic prevention. J. Pediatr. Gastroenterol. Nutr., 68 (1), 17−19. doi:10.1097/MPG.0000000000002113.A
175 Piątek, J. et al. (2021) Effects of a nine-strain bacterial synbiotic compared to simethicone in colicky babies – An open-label randomised study. Benef. Microbes, 12 (3), 249−257. doi:10.3920/BM2020.0160
176 Kianifar, H. et al. (2014) Synbiotic in the management of infantile colic: A randomised controlled trial. J. Paediatr. Child. Health, 50 (10), 801−805. doi:10.1111/jpc.12640
177 Huffman, F. G. & Walgren, M. E. (2003) L-Glutamine supplementation improves nelfinavir-associated diarrhea in HIV-infected individuals. HIV Clin. Trials, 4 (5), 324−329. doi:10.1310/BFDT-J2GH-27L7-905G
178 Field, A. S. & Milner, D. A. (2015) Intestinal microsporidiosis. Clin. Lab. Med., 35 (2), 445−459. doi:10.1016/j.cll.2015.02.011
179 Salminen, M. et al. (2004) The efficacy and safety of probiotic Lactobacillus rhamnosus GG on prolonged, noninfectious diarrhea in HIV patients on antiretroviral therapy: a randomized, placebo-controlled, crossover study. HIV Clin. Trials, 5 (4), 183−191.
180 Wolf, B. W., Wheeler, K. B., Ataya, D. G. & Garleb, K. A. (1998) Safety and tolerance of Lactobacillus reuteri supplementation to a population infected with the human immunodeficiency virus. Food Chem. Toxicol., 36 (12), 1085−1094. doi:10.1016/S0278-6915(98)00090-8
181 Balta, I. et al. (2015) Insulin resistance in patients with post-adolescent acne. Int. J. Dermatol., 54 (6), 662−666. doi:10.1111/ijd.12426
182 Nagpal, M., De, D., Handa, S., Pal, A. & Sachdeva, N. (2016) Insulin resistance and metabolic syndrome in young men with acne. JAMA Dermatol., 152 (4), 399−404. doi:10.1001/jamadermatol.2015.4499
183 Fabbrocini, G., Bertona, M., Picazo, Pareja-Galeano, H., Monfrecola, G. & Emanuele, E. (2016) Supplementation with Lactobacillus rhamnosus SP1 normalises skin expression of genes implicated in insulin signalling and improves adult acne. Benef. Microbes, 7 (5), 625−630. doi:10.3920/BM2016.0089
184 Dzidic, M. et al. (2017) Aberrant IgA responses to the gut microbiota during infancy precede asthma and allergy development. J. Allergy Clin. Immunol., 139 (3), 1017−1025.e14. doi:10.1016/j.jaci.2016.06.047
185 Clark, H. et al. (2019) Differential associations of allergic disease genetic variants with developmental profiles of eczema, wheeze and rhinitis. Clin. Exp. Allergy, 49 (11), 1475−1486. doi:10.1111/cea.13485
186 Korpela, K. et al. (2018) Probiotic supplementation restores normal microbiota composition and function in antibiotic-treated and in caesarean-born infants. Microbiome, 6 (1), 1−11. doi:10.1186/s40168-018-0567-4
187 Johansson, M. A., Sjögren, Y. M., Persson, J. O., Nilsson, C. & Sverremark-Ekström, E. (2011) Early colonization with a group of Lactobacilli decreases the risk for allergy at five years of age despite allergic heredity. PLoS One, 6 (8), 1−8. doi:10.1371/journal.pone.0023031
188 Björkander, S. et al. (2020) Childhood allergy is preceded by an absence of gut lactobacilli species and higher levels of atopy-related plasma chemokines. Clin. Exp. Immunol., 202 (3), 288−299. doi:10.1111/cei.13494
189 Lundelin, K., Poussa, T., Salminen, S. & Isolauri, E. (2017) Long-term safety and efficacy of perinatal probiotic intervention: Evidence from a follow-up study of four randomized, double-blind, placebo-controlled trials. Pediatr. Allergy Immunol., 28 (2), 170−175. doi:10.1111/pai.12675
190 Tan, W., Zhou, Z., Li, W., Lu, H. & Qiu, Z. (2021) Lactobacillus rhamnosus GG for cow’s milk allergy in children: a systematic review and meta-analysis. Front. Pediatr., 9, 727127. doi:10.3389/fped.2021.727127
191 Canani, R. B. et al. (2016) Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. ISME J., 10 (3), 742−750. doi:10.1038/ismej.2015.151
192 Paparo, L. et al. (2019) Randomized controlled trial on the influence of dietary intervention on epigenetic mechanisms in children with cow’s milk allergy: the EPICMA study. Sci. Rep., 9 (1), 1−10. doi:10.1038/s41598-019-38738-w
193 Piirainen, L., Haahtela, S., Helin, T., Korpela, R., Haahtela, T. & Vaarala, O. (2008) Effect of Lactobacillus rhamnosus GG on rBet v1 and rMal d1 specific IgA in the saliva of patients with birch pollen allergy. Ann. Allergy, Asthma Immunol., 100 (4), 338−342. doi:10.1016/S1081-1206(10)60596-0
194 Moreira, A., Kekkonen, R., Korpela, R., Delgado, L. & Haahtela, T. (2007) Allergy in marathon runners and effect of Lactobacillus GG supplementation on allergic inflammatory markers. Respir. Med., 101 (6), 1123−1131. doi:10.1016/j.rmed.2006.11.015
195 Nutten, S. (2015) Atopic dermatitis: Global epidemiology and risk factors. Ann. Nutr. Metab., 66, 8−16. doi:10.1159/000370220
196 Watanabe, S. et al. (2003) Differences in fecal microflora between patients with atopic dermatitis and healthy control subjects. J. Allergy Clin. Immunol., 111 (3), 587−591. doi:10.1067/mai.2003.105
197 West, C. E., Rydén, P., Lundin, D., Engstrand, L., Tulic, M. K. & Prescott, S. L. (2015) Gut microbiome and innate immune response patterns in IgE-associated eczema. Clin. Exp. Allergy, 45, 1419−1429. doi:10.1111/cea.12566
198 Szajewska, H. & Horvath, A. (2018) Lactobacillus rhamnosus GG in the primary prevention of eczema in children: A systematic review and meta-analysis. Nutrients, 10 (9), 1319. doi:10.3390/nu10091319
199 Avershina, E. et al. (2017) Effect of probiotics in prevention of atopic dermatitis is dependent on the intrinsic microbiota at early infancy. J. Allergy Clin. Immunol., 139 (4), 1399−1402.e8. doi:10.1016/j.jaci.2016.09.056
200 Wu, Y. J. et al. (2017) Evaluation of efficacy and safety of Lactobacillus rhamnosus in children aged 4–48 months with atopic dermatitis: An 8-week, double-blind, randomized, placebo-controlled study. J. Microbiol. Immunol. Infect., 50 (5), 684−692. doi:10.1016/j.jmii.2015.10.003
201 Grüber, C. et al. (2007) Randomized, placebo-controlled trial of Lactobacillus rhamnosus GG as treatment of atopic dermatitis in infancy. Allergy Eur. J. Allergy Clin. Immunol., 62 (11), 1270−1276. doi:10.1111/j.1398-9995.2007.01543.x
202 Tan-Lim, C. S. C., Esteban-Ipac, N. A. R., Recto, M. S. T., Castor, M. A. R., Casis-Hao, R. J. & Nano, A. L. M. (2021) Comparative effectiveness of probiotic strains on the prevention of pediatric atopic dermatitis: A systematic review and network meta-analysis. Pediatr. Allergy Immunol., 32 (6), 1255−1270. doi:10.1111/pai.13514
203 Schmidt, R. M. et al. (2019) Probiotics in late infancy reduce the incidence of eczema: A randomized controlled trial. Pediatr. Allergy Immunol., 30 (3), 335−340. doi:10.1111/pai.13018
204 Tomic-Canic, M., Burgess, J. L., O’Neill, K. E., Strbo, N. & Pastar, I. (2020) Skin microbiota and its interplay with wound healing. Am. J. Clin. Dermatol., 21 (s1), 36−43. doi:10.1007/s40257-020-00536-w
205 Valdéz, J. C., Peral, M. C., Rachid, M., Santana, M. & Perdigón, G. (2005) Interference of Lactobacillus plantarum with Pseudomonas aeruginosa in vitro and in infected burns: The potential use of probiotics in wound treatment. Clin. Microbiol. Infect., 11 (6), 472−479. doi:10.1111/j.1469-0691.2005.01142.x
206 Peral, M. C., Huaman Martinez, M. A. & Valdez, J. C. (2009) Bacteriotherapy with Lactobacillus plantarum in burns. Int. Wound J., 6 (1), 73−81. doi:10.1111/j.1742-481X.2008.00577.x
207 Salem, I., Ramser, A., Isham, N. & Ghannoum, M. A. (2018) The gut microbiome as a major regulator of the gut-skin axis. Front. Microbiol., 9, 1459. doi:10.3389/fmicb.2018.01459
208 Mayes, T., Gottschlich, M. M., James, L. E., Allgeier, C., Weitz, J. & Kagan, R. J. (2015) Clinical safety and efficacy of probiotic administration following burn injury. J. Burn Care Res., 36 (1), 92−99. doi:10.1097/BCR.0000000000000139
209 Loesch, W. (1986) Role of Streptococcus mutans in human dental decay. Am. Soc. Microbiol., 50 (4), 353−380. doi:10.1080/11026480260363242
210 Meurman, J. H., Antila, H. & Salminen, S. (1994) Recovery of lactobacillus strain GG (ATCC 53103) from saliva of healthy volunteers after consumption of yoghurt prepared with the bacterium. Microb. Ecol. Health Dis., 7 (6), 295−298. doi:10.3109/08910609409141368
211 Nase, L. et al. (2001) Effect of long-term consumption of a probiotic bacterium, Lactobacillus rhamnosus GG, in milk on dental caries and caries risk in children. Caries Res., 35 (6), 412−420. doi:10.1159/000047484
212 Davidson, L., Fiorino, A. M., Snydman, D. & Hibberd, P. (2011) Lactobacillus GG as an immune adjuvant for live attenuated influenza vaccine in healthy adults: a randomized double blind placebo controlled trial. Eur. J. Clin. Nutr., 65 (4), 501−507. doi:10.1038/ejcn.2010.289
213 Carey, I., Critchley, J., DeWilde, S., Harris, T., Hosking, F. & Cook, D. (2018) Risk of infection in Type 1 and Type 2 diabetes compared with the general population: a matched cohort study. Diabetes Care, 41 (3), 513−521. doi:10.2337/dc17-2131/-/DC1.
214 Goeijenbier, M. et al. (2017) Benefits of flu vaccination for persons with diabetes mellitus: A review. Vaccine, 35 (38), 5095−5101. doi:10.1016/j.vaccine.2017.07.095
215 Bianchini, S. et al. (2020) Effects of probiotic administration on immune responses of children and adolescents with type 1 diabetes to a quadrivalent inactivated influenza vaccine. Hum. Vaccines Immunother., 16 (1), 86−94. doi:10.1080/21645515.2019.1633877
216 De Vrese, M., Rautenberg, P., Laue, C., Koopmans, M., Herremans, T. & Schrezenmeir, J. (2005) Probiotic bacteria stimulate virus-specific neutralizing antibodies following a booster polio vaccination. Eur. J. Nutr., 44 (7), 406−413. doi:10.1007/s00394-004-0541-8
217 Lazarus, R. P. et al. (2018) The effect of probiotics and zinc supplementation on the immune response to oral rotavirus vaccine: A randomized, factorial design, placebo-controlled study among Indian infants. Vaccine, 36 (2), 273−279. doi:10.1016/j.vaccine.2017.07.116
218 Hansen, A. (2019) Generally Recognized as Safe (GRAS) Determination for the Intended Use of Lactobacillus Rhamnosus LGG, No. 845. doi:10.1007/s40278-019-57906-7
219 Land, M. H., Rouster-Stevens, K., Woods, C. R., Cannon, M. L., Cnota, J. & Shetty, A. K. (2005) Lactobacillus sepsis associated with probiotic therapy. Pediatrics, 115 (1), 178−181. doi:10.1542/peds.2004-2137
220 Husni, R. N., Gordon, S. M., Washington, J. A. & Longworth, D. L. (1997) Lactobacillus bacteremia and endocarditis: Review of 45 cases. Clin. Infect. Dis., 25 (5), 1048−1055. doi:10.1086/516109
221 Database NM. Drug Nutrient Interaction Checker. https://naturalmedicines.therapeuticresearch.com/databases/food,-herbs-supplements/professional.aspx?productid=767.
222 Sybesma, W., Molenaar, D., van IJcken, W., Venema, K. & Korta, R. (2013) Genome instability in Lactobacillus rhamnosus GG. Appl. Environ. Microbiol., 79 (7), 2233−2239. doi:10.1128/AEM.03566-12
i Segers, M. E. & Lebeer, S. (2014) Towards a better understanding of Lactobacillus rhamnosus GG−host interactions. Microb. Cell Fact., 13 (Suppl 1), S7. doi: 10.1186/1475-2859-13-S1-S7.
ii Kankainen, M. et al. (2009) Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human-mucus binding protein. Proc. Natl Acad. Sci. USA, 106 (40), 17 193−17 198.