By Karin Elgar
Abstract
Introduction
Food sources
Bioavailability of supplements
General mechanisms
Clinical uses
Safety
Possible contamination
Drug interactions
Children
Pregnancy / lactation
Conclusion
Acknowledgements
References
Abstract
Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are omega-3 polyunsaturated fatty acids found in oily fish. Humans can also synthesise them from α-linolenic acid (ALA), which is considered an essential fatty acid as humans cannot synthesise it. However, conversion of ALA to EPA and DHA tends to be low, with significant inter-individual variation, making them conditionally essential nutrients. The benefits of EPA and DHA for cardiovascular health were first recognised in the 1970s. Since then, research has shown benefits in many other conditions, including metabolic, inflammatory and neuropsychiatric disorders, based on their anti-inflammatory and antioxidant effects, as well as their roles in cell membrane structure and function and in regulating gene expression. Fish oil supplements are generally well tolerated, but increased risk of atrial fibrillation and bleeding have been found in several meta-analyses.
Cite as: Elgar, K. (2022) EPA/DHA: A Review of Clinical Use and Efficacy. Nutr. Med J., 1 (2), 97-132.
Affiliation: K. Elgar is with the Nutritional Medicine Institute, London, UK.
Article history: Received 24 July 2021; Peer-reviewed and received in revised form 25 September 2021; Accepted 08 October 2021. Available online 31 May 2022.
Published by: The Nutritional Medicine Institute
Open Access: This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs licence (http:// creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reproduction and distribution of the work, in any medium, provided the original work is not altered or transformed in any way, and that the work is properly cited. For commercial use please contact support@nmi.health
Introduction
Since the finding in the early 1970s that Eskimos from Greenland had low rates of heart disease despite a high-fat diet, but a diet high in marine omega-3 polyunsaturated fatty acids (PUFAs),1 there has been significant interest in the potential benefits of fish and fish oils for health, in particular cardiovascular (CV) health.
Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are omega-3 (n-3) PUFAs, fatty acids with more than one cis double-bond.2 n-3 indicates that the first double-bond is located between the third and fourth carbon atoms counting from the methyl end of the molecule, whilst omega-6 (n-6) PUFAs have the first double-bond between the sixth and seventh carbon atoms.
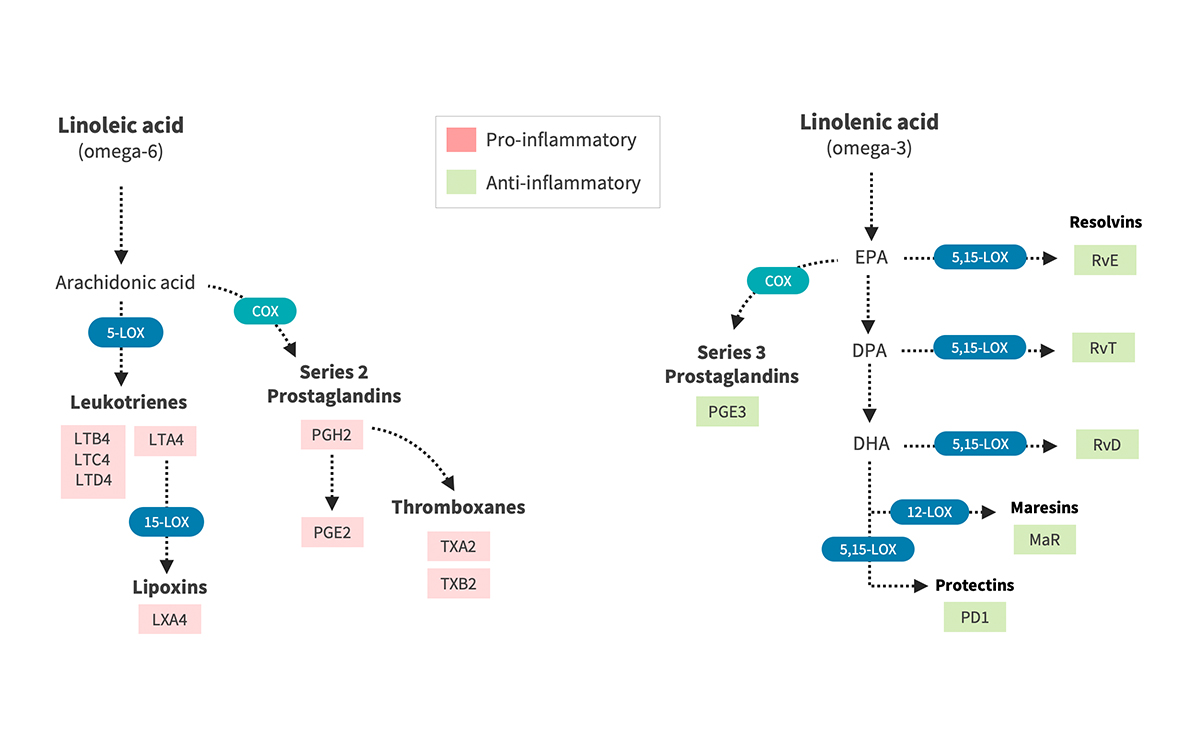
The n-3 PUFA α-linolenic acid (ALA) and the n-6 PUFA linoleic acid (LA) are considered essential fatty acids, as humans cannot synthesise them, and food sources include green vegetables, flax and chia seeds and nuts, and vegetable oils, respectively. These parent PUFAs can be converted to EPA and from there to DHA and the n-6 long-chain fatty acids gamma-linolenic acid (GLA) and arachidonic acid (AA) (see figure 1).
Figure 1: Synthesis of Eicosanoids and Lipid Mediators from Essential Fatty Acids
Figure 1: Dietary omega-6 fatty acids are precursors of arachidonic acid (left), which gives rise to pro-inflammatory leukotrienes, series 2 prostaglandins and thromboxanes. Conversely, omega-3 fatty acids (EPA, DPA and DHA) are precursors of various anti-inflammatory series 3 prostaglandins and specialized pro-resolving mediators (resolvins, maresins and protectins), which participate in the resolution of inflammation. Abbreviations: LOX, lipoxygenase; COX, cyclooxygenase; Rv, resolvins; PD1, protectin D1; MaR, maresins). Illustration by Kelly Heim, Ph.D., IntegrativePharmacology.com.
However, conversion rates tend to be low, with up to 8% conversion of ALA to EPA in men and up to 21% in women, making them conditionally essential nutrients.3 Apart from these gender differences, conversion can also vary significantly between individuals based on genetic variations in the enzymes involved,4 and is known to be decreased (leading to low EPA levels) in certain chronic conditions, including diabetes.5 The enzymes responsible for conversion also require vitamins and minerals, including magnesium, zinc, B vitamins and vitamin C, as co-factors, therefore nutrient deficiencies may also affect conversion.6
The n-3 PUFA status is often expressed as the Omega-3 Index (O3I), which is a measure of the percentage of EPA and DHA of all fatty acids in the membranes of red blood cells (RBCs).7 This measure was first established to assess risk of death from coronary heart disease (CHD), and the optimal O3I is thought to be 8−12%, with < 4% conferring a significantly increased risk.8 Levels below 2% have not been found, suggesting that this is the minimum level to support life.9 Low O3I has also been associated with many other conditions, as discussed under the section ‘Clinical uses’.
Not all clinical trials established O3I at baseline, which may explain the sometimes contradictory results. A 2019 study set out to establish a model that would allow to estimate the change in O3I depending on supplemental dose.7 The authors took data from 14 studies (1422 individuals) and found that after dose, baseline O3I and type of supplement were the most important determinants of an increase in O3I, with triglyceride forms raising O3I more than ethyl esters (EEs). The model suggests that to increase O3I from below 4% to above 8%, 95% of people would need about 2000 mg EPA and DHA combined per day, which is significantly more than the amount found in the one−two portions of seafood per week recommended by the American Heart Association. The authors note that whilst this model would be useful on a population basis, in clinical practice direct testing of O3I would be preferable due to the significant inter-individual variability in response to supplementation.7 In one study of 40 participants with an O3I < 5%, the change in O3I after 8 weeks of consuming a drink containing EPA 200 mg and DHA 300 mg per day ranged from −0.03% to +7.16%.10
Just as important as absolute intakes and levels of n-3 PUFAs is the ratio of n-6 versus n-3 PUFAs, as the same enzymes are responsible for conversion of the parent PUFAs to EPA, DHA, GLA and AA, respectively. In a typical Western diet, the n-6:n-3 ratio tends to be very high, in the range of 10:1 to 25:1, whilst it is believed that our ancestor hunter−gatherers consumed a diet with a ratio nearer to 1:1, a change that is thought to be implicated in the high prevalence of inflammatory conditions, including cardiovascular disease (CVD).11,12 It has also been suggested that lowering dietary n-6 may have a positive effect on n-3 status.13
Food sources
Oily fish, such as salmon, mackerel and sardines, is the richest dietary source of EPA and DHA.14 Eggs from hens fed a high n-3 diet are a good source of DHA,15 and meat from grass-fed (pastured) cattle and lamb also provide EPA and DHA16,17,18 (see Table 1 for contents of EPA and DHA in foods).
Table 1: Food sources of EPA and DHA
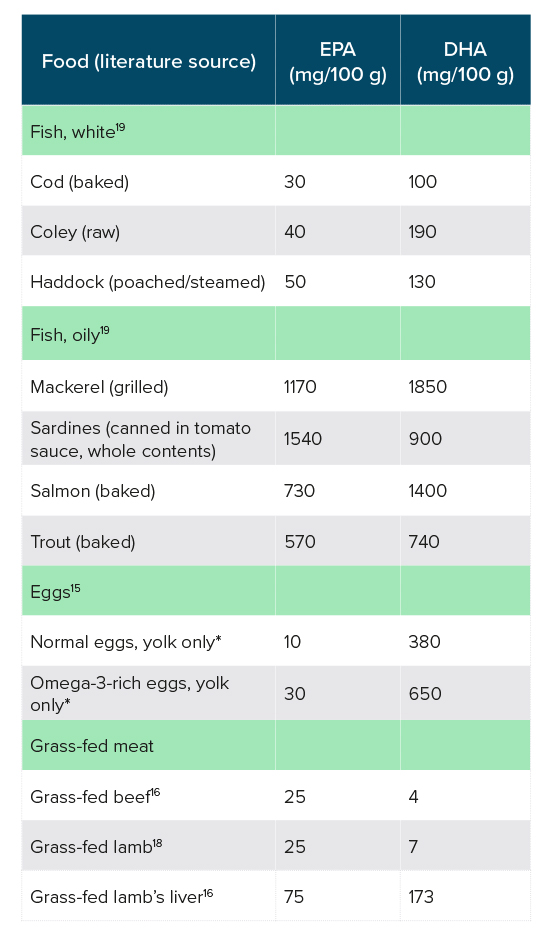
*An egg contains approximately 18 g yolk.
The Scientific Advisory Committee on Nutrition (SCAN) recommends a daily intake of 450 mg EPA and DHA combined.20 From the data in Table 1, it appears that a well-planned omnivorous or pescatarian diet, including oily fish 1−2 times per week, can provide this level of EPA and DHA. However, in 2001/2002, the average consumption of EPA and DHA in the UK was 244 mg per day in adults, 199 mg of which came from fish.20 SCAN concludes that “The majority of the UK population does not consume enough fish, particularly oily fish, … the COMA population guideline recommendation that people should eat at least two portions of fish a week of which one should be oily. Consumption of this amount would probably confer significant public health benefits to the UK population in terms of reducing CVD risk. There may also be beneficial effects on fetal development”.21
Reasons for the low consumption of fish may include taste and dietary preferences, cultural/religious reasons, cost and availability, as well as concerns over sustainability, and contamination with heavy metals and other toxins. Most red meat consumed in the UK comes from grain-fed animals, which has much lower levels of EPA and DHA and higher levels of AA.22
A cross-sectional study in the USA and Canada showed that among people not taking fish oil supplements, only 3% of those consuming 1 fish serving per week and 17% consuming ≥ 2 servings achieved an optimal O3I of ≥ 8.0%.23 The study also suggested that non-fish eaters would need to supplement 1300 mg per day of EPA and DHA to achieve an optimal O3I. The large variability in n-3 metabolism and response to intake needs to be taken into account though; in a clinical setting RBC O3I should ideally be determined at baseline to establish need, and then used to monitor response to dietary/supplement intervention.
Overall, supplementation may be necessary to make up for dietary shortfalls, and will also be necessary where high dosages are desirable for therapeutic reasons (see the section ‘Clinical uses’).
The DHA can be retro-converted to EPA,3 making DHA supplements of algal origin a viable option for n-3 supplementation for vegetarians and vegans. It is important to note that both dietary intake and individual metabolism of PUFAs determine a person’s PUFA status.4
Bioavailability of supplements
Apart from differing ratios of EPA and DHA in supplements, which are discussed in the section ‘Clinical uses’ where relevant, fish oils also come in various different formulations, mostly EEs, free fatty acids (FFAs) and triglycerides/triacyl glycerides (TGs). A number of studies have looked at the bioavailability of these formulations.
Whilst some studies found TG formulations to have better bioavailability than EE,24,25,26 others have found no difference between the two.27,28,29,30 Studies that compared FFAs with EE formulations found significantly better bioavailability of the FFA supplements, especially when administered with a low-fat meal.26,31,32,33 This is thought to be due to the fact that lipase is needed for digestion of the EE form.32 FFA fish oils have also been shown to be better absorbed than TG formulations.26 The absorption of FFAs appears to be similar to that of naturally occurring fish oils, as in fish body or cod liver oil.31
Overall, the evidence suggests that fish oils in the FFA form have the best bioavailability, especially when taken with a low-fat meal. It should be noted that much of the research into bioavailability of fish oils was carried out in the 1990s.
The aim of this paper is to review the evidence for benefits of EPA and DHA supplements from clinical intervention studies.
General mechanisms
Membrane structure and function
The n-3 and n-6 PUFAs are essential structural components of cell membranes. The double-bonds in the carbon-chain of fatty acids introduce ‘kinks’, which affect the fluidity of the membrane’s phospholipid-bilayer and as such its permeability, as well as the structure and function of membrane-bound proteins (including enzymes and ion channels) and cell signalling pathways.34,35,36 Dietary intake of PUFAs can therefore play an important role in membrane structure and function.
The predominant PUFA in retinal and neuronal postsynaptic cell membranes is DHA, and as such is thought to play an important role in eye and nervous system function and development of the foetus.35,36
Anti-inflammatory effects
EPA and DHA are probably most used for their anti-inflammatory properties, which are mediated through a number of mechanisms.
In response to hormones, cytokines (inflammatory mediators) and other compounds, PUFAs can be released from cell membranes and converted to a range of eicosanoids that can have either pro- or anti-inflammatory properties, as well as regulate vascular permeability, vasoconstriction and bronchoconstriction.36 The interplay of these inflammatory mediators is complex but, generally speaking, eicosanoids derived from n-3 PUFAs are considered to be more anti-inflammatory.36
EPA and DHA are also precursors to specialised pro-resolving mediators, compounds that ensure that inflammation, which is an essential protective and repair mechanism, is switched off after the acute inflammation following an insult, such as injury or infection, and does not turn into chronic inflammation, which is the main underlying cause of most chronic diseases.37
Another anti-inflammatory mechanism of EPA and DHA is via regulation of gene expression (see below).
Over 20 randomised-controlled trials (RCTs) of fish oil have collected data on inflammatory markers, and several meta-analyses have concluded that EPA and DHA can reduce C-reactive protein (CRP; a marker of inflammation) in people with dyslipidaemia and/or high CRP levels at baseline,38 in patients with HIV,39 and in patients on haemodialysis,40 whilst they found no effects on tumour necrosis factor-alpha (TNF-α) or interleukin-6 (IL-6),39,40 two other commonly measured inflammatory markers.
Antioxidant effects
The n-3 fatty acids are also believed to have antioxidant properties, although some studies in the 1990s have actually shown pro-oxidant effects at high dosages. This is thought to be due to the high degree of unsaturation (i.e. double-bonds between the carbon atoms) that makes them unstable and prone to oxidation. Studies have shown benefits of combinations of omega-3 PUFAs with vitamin E, which acts as an antioxidant and thus protects the n-3 fats.41
For example, malondialdehyde (MDA; a marker of oxidative stress) and lipid peroxides (oxidised lipids) increased in a double-blind, placebo-controlled trial of a high-dose fish oil supplement, EPA 3062 mg and DHA 2262 mg per day, with tert-butylhydroquinone (a synthetic phenol used as a preservative).42 This increase was seen both with and without additional vitamin E (900 IU per day as DL-α-tocopheryl acetate). A study using 10 000 mg fish oils per day for 4 weeks also found increases in oxidised plasma lipoproteins both in smokers and non-smokers, which was ameliorated when vitamin E (600 IU per day) was added.43
However, whilst there is some conflicting research on this topic, two recent meta-analyses do not confirm a pro-oxidant effect of EPA and DHA.41,44 A meta-analysis of 39 studies found that EPA and DHA supplements can significantly increase total antioxidant capacity (TAC) and activity of glutathione peroxidase (an antioxidant enzyme), and decrease MDA, whilst it found no significant effects on reduced glutathione, nitric oxide (NO), or the antioxidant enzymes superoxide dismutase (SOD) and catalase (CAT).44 Similarly, a meta-analysis of nine RCTs looking at co-supplementation of vitamin E and n-3 fatty acids (from plant or animal origin) found significant improvements of MDA, TAC as well as NO, but not glutathione, SOD or CAT.41 Neither study found a worsening of any oxidative stress markers.
Whilst the evidence from clinical trials supports an antioxidant, rather than pro-oxidant, effect of EPA and DHA, it should be borne in mind that these fats are vulnerable to oxidation, and the antioxidant status of the person taking fish oils needs to be considered.
Regulation of gene expression
The n-3 PUFAs have been shown to regulate gene expression for proteins involved in lipid and carbohydrate metabolism, thermogenesis and inflammatory processes by binding to transcription factors (proteins that control the transcription of genes by binding to specific DNA sequences), including sterol regulatory element-binding protein, peroxisome proliferator-activated receptors, carbohydrate response element-binding protein and nuclear factor-kappa B.36,45
Clinical uses
Attention deficit hyperactivity disorder (ADHD)
Attention deficit hyperactivity disorder is characterised by a variety of symptoms, including hyperactivity, restlessness, inability to stay focused, mood swings, temper tantrums, problems completing tasks and impulsivity, and is commonly associated with other disorders including anxiety, depression and learning difficulties.46
A review of 15 observational studies looking at n-3 levels in people with ADHD has found lower O3I compared with healthy controls, which could not consistently be explained with dietary intakes, suggesting metabolic differences to be responsible for this finding.46 The authors suggest that measuring baseline O3I may help identify those patients who may benefit most from fish oil supplementation. A more recent observational study from Italy confirms these findings, reporting a lower O3I in children with ADHD than in healthy controls.47
A 2018 meta-analysis of n-3 monotherapy RCTs in children with ADHD found significant improvements in ADHD clinical symptom scores (based on five trials) and cognitive measures associated with attention (based on three trials).48 Dosages used in the trials included in this meta-analysis ranged from 126 to 1290 mg combined EPA and DHA per day. Except for two studies that only used EPA, all studies used combinations of EPA and DHA, at various ratios. Durations were not reported. Another meta-analysis including 10 RCTs in children or adults found no benefits of fish oils when pooling data from all studies, but significant improvements in emotional liability and oppositional behaviour when limiting the meta-analysis to high-quality studies.49 Dosages and durations were not reported.
Significant improvements have also been seen with EPA 294 mg and DHA 206 mg per day for 12 weeks, alongside a Korean red ginseng extract.50,51
Overall, the clinical evidence suggests a benefit of EPA and DHA for children with ADHD, with a range of dosages showing benefits.
Asthma
Asthma is a chronic inflammatory disease of the airways, and is associated with airway hyper-responsiveness, airway smooth muscle contraction and excess mucous production.52
A cross-sectional study in Korean pre-school children showed that children with atopic conditions (asthma, allergic rhinitis and/or atopic dermatitis) had lower O3I than children without atopy, 7.8% versus 9.1%.53 They also had lower overall n-3 PUFAs, and higher n-6 PUFAs and n-6:n-3 ratio. An Australian cross-sectional study on the other hand found no difference in O3I in adults with and without asthma, 6.2% versus 6.1%, and in fact showed higher n-3 PUFAs and a lower n-6:n-3 ratio in asthmatics compared with healthy controls.52 They did, however, find that those asthmatics with the higher O3I had better controlled asthma.
A 2002 Cochrane review found no consistent effects of fish oils on markers of asthma severity, including forced expiratory volume (FEV1), peak flow rate, asthma symptoms, asthma medication use or bronchial hyperreactivity.54 Adult dosages ranged from 1000 to 5400 mg combined EPA and DHA, at varying ratios, and durations ranged from 8 weeks to 12 months.
Since then, several more studies have evaluated the use of fish oils in asthma with contradictory results.
A double-blind, placebo-controlled trial in 12−25 year olds with overweight/obesity and uncontrolled asthma found no benefit of fish oils (EPA 3180 mg and DHA 822 mg per day) for 24 weeks.55 A double-blind, placebo-controlled study investigating a combination of fish oils (EPA 230 mg, DHA 125 mg per day), probiotics, and a fruit and vegetable extract in children aged 10−12 years, on the other hand, found significant improvements in pulmonary function and reduced medication use compared with placebo, although there was no difference in Paediatric Asthma Quality of Life Questionnaire score and the Childhood Asthma Control Test.56 Because this was a multi-nutrient intervention, the effect of the fish oils cannot be determined.
In adults, a double-blind, crossover study found significant benefits for exercise-induced bronchoconstriction at a dose of EPA 3200 mg and DHA 2000 mg per day for 3 weeks.57 On the other hand, another double-blind, crossover study, using slightly higher doses (EPA 4000 mg and DHA 2000 mg per day, for 3 weeks), found no benefits despite an observed change in blood fatty acids confirming compliance of study subjects.58
A couple of uncontrolled/unblinded studies found benefits of fish oils, EPA 180 mg and DHA 120 mg per day for 6 and 3 months, respectively, in adults59 and children.60
Overall, the results from clinical research are contradictory. Long-term supplementation (3−6 months) with a low dose, for example EPA 180 mg and DHA 120 mg per day, appears to be of more benefit than high-dose supplementation, although the current evidence is insufficient to make clear recommendations on supplementation of fish oils in asthmatics.
Atopic dermatitis
Atopic dermatitis/eczema is a chronic inflammatory condition of the skin.
A couple of small double-blind, placebo-controlled trials showed significant benefits in patients with eczema. One study with 23 participants used EPA 1800 mg and DHA 1200 mg per day for 12 weeks, and found significant improvements of patient-assessed itch and scale and total score with fish oil versus placebo, whilst physician-assessed scores improved by 30%, but this failed to reach statistical significance.61 Another study in 53 patients found that DHA 5350 mg and EPA 370 mg per day for 8 weeks led to a statistically significant decrease in severity scoring of atopic dermatitis (SCORAD) from 37 to 28.5, compared with a non-significant reduction with placebo from 35.4 to 33.62
Whilst research into the benefits of fish oils in atopic dermatitis is very limited, there are some promising results. Both studies used high doses of fish oils but very different EPA:DHA ratios, making it difficult to make any particular recommendations.
Autism
Autism spectrum disorder (ASD) is characterised by difficulties in social communication and interaction, and restricted and/or repetitive interests, activities and/or patterns of behaviour.63 PUFAs, in particular EPA and DHA, have received much interest in the treatment of ASD due to their roles in brain functioning through their anti-inflammatory properties and importance for brain cell membrane integrity.63 Restricted dietary behaviours in individuals with ASD may lead to nutrient deficiencies, including EPA and DHA.
One study looked at O3I of children with ASD and found a low O3I of 4.8% at baseline, which increased by 4.2% in those who received DHA.64
Three meta-analyses have reviewed the clinical evidence for the use of n-3 PUFAs in ASD over the past 5 years, including basically the same studies and coming to similar conclusions.63,65,66 The most comprehensive and recent article reviewed 13 RCTs, and included nine trials, involving 372 children, in the meta-analysis.65 It found a significant improvement with n-3 supplementation in the overall Aberrant Behaviour Checklist (ABC) score, but not in the ABC subscores or the Social Responsiveness Scale. Dosages ranged from 200 mg (DHA only) to 1500 mg of EPA and DHA combined, with various ratios of EPA and DHA. Some formulations also included n-6 fatty acids and/or vitamins and minerals. The durations of the studies ranged from 6 to 24 weeks. Eight out of the 13 studies found significant improvements in some outcome measures, but there was no obvious correlation with either dose or EPA:DHA ratio.
Two more double-blind RCTs have been published since. One RCT, using EPA 180 mg and DHA 120 mg per day for 8 weeks, found small but statistically significant improvements in stereotyped behaviour, social communication and Gilliam Autism Rating Scale, but no change in social interaction.67 The other study used DHA (722 mg per day) for 12 months, and reported significant improvements in inappropriate speech, stereotypy, lethargy and irritability compared with placebo.64
Overall, there appears to be a benefit of EPA and DHA in ASD, but the data are too inconsistent to make recommendations regarding dosage or EPA:DHA ratio.
Autoimmune conditions
Inflammatory bowel disease (IBD)
Inflammatory bowel disease is an auto-inflammatory condition characterised by inflammation of the gastrointestinal tract (GIT), and includes Crohn’s disease (CD; which can affect any part of the GIT) and ulcerative colitis (UC; which only affects the colon). Due to the inflammatory nature of these conditions, fish oil supplements have received much interest because of their anti-inflammatory effects.
A study in patients with CD has shown that n-3 levels in serum were half those of healthy controls.68 This study also showed that supplementation with EPA 510 mg and DHA 344 mg per day alongside vitamin D (1000 IU per day; vitamin D is commonly low in patients with IBD) for 4−6 weeks increased n-3 status, although this did not have any effect on CRP or calprotectin (a marker of intestinal inflammation).68
By far the largest clinical trials into the potential benefits of fish oils in preventing relapse in CD are the Epanova Program in Crohn’s (EPIC) Studies 1 and 2.69 These double-blind, placebo-controlled trials of 363 and 375 patients, respectively, investigated the effects of EPA 2000 mg and DHA 600 mg for up to 58 weeks, and found no significant reduction in relapse rate compared with placebo. An RCT in children with CD, using EPA 400 mg and DHA 200 mg per day, on the other hand found a significant reduction of relapses over a 1-year period, 95% in the placebo group compared with 61% in the fish oil group.70
One RCT compared n-3 (3000 mg per day, dose of EPA and DHA not reported) versus n-6 supplementation (7600 mg per day), and found a decrease in CD Activity Index and CRP in both groups (all patients also received other medications).71 Whilst the n-3 group had stable pro- and anti-inflammatory markers over the 2 months of supplementation, despite a lower than usual dose of steroids, the n-6 groups had a significant increase in pro- and decrease in anti-inflammatory cytokines.
Although evidence is somewhat mixed, at present the clinical research does not support a recommendation of fish oil supplements for patients with CD.
Findings from clinical trials in UC, mostly from the 1990s, are also mixed. A number of double-blind, placebo-controlled trials have found benefits for patients with UC with regards to disease markers such as calprotectin72 and leukotriene B4 (an inflammatory marker),73,74 as well as clinical outcomes, such as disease activity index and/or histological findings,72,73 and reduction in medication use.73,74 However, other double-blind/placebo-controlled trials found no significant benefits,75,76 sometimes despite an improvement in n-3 status.76
A decrease in inflammatory and/or oxidative stress markers in patients with UC has also been seen in a number of unblinded and/or uncontrolled trials,77,78,79,80,81 although these were not always associated with significant clinical improvements.77,78,79,80
Most of these trials used high dosages of EPA and DHA, combined dose 4100−6400 mg per day or EPA alone 2000−4500 mg per day, and durations ranged from 3 months to 2 years. There was no obvious trend of either a combination of EPA and DHA or EPA alone, or dose or duration to affect results.
An open-label pilot study, using EPA 2000 mg per day, looked at changes in the microbiome, which is commonly abnormal in patients with UC, and found improvements after 3 months, which were accompanied by clinical improvements.82
Although results are mixed, there appears to be some benefit of EPA and DHA in UC but, at this point, there are insufficient data to recommend a particular dose. Assessing O3I at baseline may help identify patients who may benefit from fish oil supplementation.
Multiple sclerosis (MS)
Multiple sclerosis is a chronic inflammatory disease characterised by demyelination of nerve cells and injury to the central nervous system.83
A 2019 meta-analysis of four double-blind RCTs found no significant effect of EPA and DHA on the Expanded Disability Status Scale (EDSS).84 This meta-analysis also looked at inflammatory markers; both studies that investigated IL-1b found significant improvements, whilst only one of two studies that investigated TNF-α and IL-6 found significant decreases. Dosages of combined EPA and DHA used in the studies included in this meta-analysis ranged from 300 to 4000 mg per day for 12−24 weeks, with various EPA:DHA ratios. Overall higher dosages appeared to give better results.
Two double-blind, placebo-controlled studies not included in the above meta-analysis have been published. One using low levels of EPA and DHA, 180 mg and 120 mg per day, respectively, for 12 months found no effect on inflammatory markers or EDSS.85 The other supplemented EPA 800 mg and DHA 1600 mg per day for 12 months, and found significant improvements in inflammatory markers, but not in the glutathione redox system, EDSS or relapse rate, despite a more than doubling in O3I (from 3.7% to 8.0%) and a concomitant decrease in the AA:EPA ratio.83,86
A small open-label study using EPA 2900 mg and DHA 1900 mg per day investigated a particular marker, matrix metalloproteinase-9 (MMP-9), which is thought to play an important role in MS by disrupting the blood−brain barrier, aiding the migration of inflammatory cells into the central nervous system.87 The fish oil supplement decreased MMP-9 after 3 months of supplementation, but did not lead to an improvement in quality of life. A 6.3-fold increase in EPA and a 1.7-fold increase in DHA in RBC membranes was also seen in this study.
Overall, the evidence suggests that EPA and DHA have a positive effect on a variety of biomarkers but not on clinical outcomes in patients with MS.
Psoriasis
Psoriasis is an autoimmune condition of the skin that is characterised by excessive proliferation of cells in the epidermis, which leads to red, flaky patches of skin covered with silvery scales.
Two meta-analyses on the use of fish oils in psoriasis were published in 2019, with differing conclusions. One meta-analysis of 10 RCTs including 560 patients found significant improvements in Psoriasis Area and Severity Index (PASI), erythema (redness) and scaling, whilst improvements in other outcome measures, itching, desquamation, infiltration and percent total body surface area, failed to reach statistical significance.88 Overall, eight out of the 10 studies reported some benefits. Dosages used in most of the included studies ranged from 352 to 6000 mg per day combined EPA and DHA, at various ratios, with durations of 4−36 weeks.
The other article reviewed 13 RCTs, but only included three in the actual meta-analysis and found no improvement in PASI.89 Of the studies not included in the meta-analysis of this review article, one used intravenous EPA and DHA, and one used a supplement mostly containing n-6 (GLA). Six of the remaining eight studies did show some positive results. Dosages and durations were in similar ranges as in the above meta-analysis.
Overall, the evidence suggests that fish oils are of benefit in psoriasis, with a wide range of dosages and EPA:DHA ratios showing positive results.
Rheumatoid arthritis (RA)
Rheumatoid arthritis is a systemic autoimmune inflammatory condition affecting the joints, but also increasing the risk of CVD in patients. RA affects 0.5−1% of the population worldwide, with women more commonly affected than men.90
A 2018 meta-analysis of 20 RCTs involving 1252 patients with RA found that fish oil supplementation significantly improved disease-related markers: early morning stiffness; tender joint count; erythrocyte sedimentation rate (a marker of inflammation); pain; Health Assessment Questionnaire; Ritchie articular index; and grip strength.90 Of five inflammatory markers evaluated, only one, leukotriene B4, was significantly reduced compared with placebo, whilst there was no significant improvement in CRP, IL-6, TNF-α and IL-1. Dosages ranged from 1670 to 5400 mg per day, one study used EPA and one ALA only, all other studies used various combinations of EPA and DHA, for 12−72 weeks. Notably, the study on ALA only did not find any benefits for RA outcomes.91
A number of RCTs not included in the above meta-analysis, many from the 1990s, have also found benefits of EPA and DHA in patients with RA92,93,94,95,96,97,98,99 and juvenile idiopathic arthritis.100
A couple of studies compared high-dose versus low-dose fish oil supplements, and found improvements in clinical outcomes with the high dose but not the low dose. Doses in these studies were EPA 728 mg and DHA 156 mg versus EPA 364 mg and DHA 78 mg per day,99 and combined EPA and DHA 5500 mg per day versus 400 mg per day.93
With an increase in veganism/vegetarianism, plant-based n-3 supplements have also received interest, and whilst ALA does not appear to have beneficial effects in RA,91 DHA from algae, 2100 mg per day for 10 weeks, has been shown in a double-blind, placebo-controlled crossover study to improve clinical outcomes and decrease both RBC AA:EPA and AA:DHA ratios, resulting in a positive shift in eicosanoids.101
Most trials used dosages of combined EPA and DHA of 3000 mg per day and more, with a range of 1300−5800 mg per day for 3−12 months. EPA:DHA ratio varied between studies.
The evidence from clinical trials is overwhelmingly in favour of a therapeutic effect of EPA and DHA in RA, with higher dosages showing better results. A daily dose of 3000 mg per day could be suggested, whilst the ratio of EPA:DHA does not appear to make a difference.
Systemic lupus erythematosus (SLE)
Systemic lupus erythematosus is a systemic autoimmune condition, affecting mostly women, which is characterised by inflammation in several tissues and organs, including joints, skin, kidneys and blood vessels, potentially causing significant damage to these organs and increasing the risk of CVD.102
A cross-sectional pilot study in 33 women with SLE, with or without concomitant CVD, and 20 healthy controls found that women with SLE had a significantly lower O3I and higher AA:EPA ratio than healthy controls, irrespective of their CVD status.103
A 2020 meta-analysis of five RCTs involving 274 patients with SLE found a significant reduction in SLE Disease Activity Index with fish oils.104 Dosages of combined EPA and DHA used in the studies ranged from 1280 to 4500 mg per day, for 12−26 weeks, with varying EPA:DHA ratios. Studies with dosages of 3000 mg per day or more appeared to show more benefits.
Three double-blind, placebo-controlled crossover studies from the 1990s, not included in the above meta-analysis, gave conflicting results. One study showed an improvement after 12 weeks in 14 out of 17 patients on the fish oil supplement (20 000 mg n-3 fats, EPA and DHA amounts and ratio not reported), whilst 13 out of 17 patients stayed the same or worsened on the placebo, a statistically significant difference.105 All patients in this study followed a low-fat diet, and also received vitamin A (4000 IU) and vitamin D (400 IU). The second study found improvements in the fish oil group (200 mg per kg body weight, DHA:EPA not reported) compared with the placebo group after 3 but not after 6 months of supplementation.106 The third study focussed on patients with stable lupus nephritis, and found no difference between fish oil, EPA 2700 mg and DHA 1700 mg per day, and placebo after 1 year of supplementation in either disease activity or renal function.107
There appears to be a benefit of EPA and DHA in SLE, at a dose of 3000 mg per day combined or more.
Cardiovascular disease
Cardiovascular disease is a general term for diseases affecting the heart and blood vessels, and includes CHD, which can lead to angina, myocardial infarction (MI) and heart failure, strokes and transient ischaemic attacks (TIAs), and peripheral artery disease. CVD is the leading cause of death globally, and it presents a significant healthcare burden.108
The n-3 PUFAs have been extensively studied in the prevention and treatment of CVD, both in clinical trials and in observational studies. There have been contradictory findings leading to controversy over the use of fish oils in patients with or at risk of CVD.
Low O3I has been associated with increased risk of CVD, and cut-off points of low (4%), intermediate (4−8%) and a desirable high level of > 8%, at which CVD risk is lowest, have been suggested in the early 2000s.109 These findings were confirmed in a 2017 meta-analysis of 10 observational studies, which also found a 15% decrease in risk of CHD for a 2.1% increase in O3I.110 Similar reductions were also found in the Framingham offspring cohort (2500 participants) where those in the highest O3I quintile (> 6.8%) had a 39% lower risk of CVD events compared with the lowest quintile (< 4.2%), and a 34% lower risk of all-cause death, although there was no statistically significant reduction in risk of CVD death.111 This study found a stronger association for DHA than EPA.
In 2018, a meta-analysis of 10 RCTs, which included at least 500 participants and lasted for at least 1 year (47 803 participants overall), made headlines as it concluded that there is no statistically significant effect of EPA and DHA on either CHD event, CHD deaths, non-fatal MI or any major vascular event.112 Dosages used in the included trials ranged from 376 to 2550 mg per day.
Since then, seven more meta-analyses of RCTs have been published, all of which show benefits of fish oils in CVD.108,113,114,115,116,117,118 The most recent and most comprehensive one reviewed 83 RCTs and cohort studies for 11 CV outcomes.113 The findings are as follows.
- Total mortality from CV causes:
– 26 RCTs with 82 696 participants: statistically significant 8% reduction in risk, low heterogeneity;
–15 cohorts with 558 826 participants: statistically significant 24% reduction, high heterogeneity.
- CV deaths:
– 22 RCTs with 76 407 participants: statistically significant 7% reduction, low heterogeneity.
- Cardiac death:
– 20 RCTs with more than 79 410 participants: statistically significant 10%
reduction, low heterogeneity.
- Post-operative atrial fibrillation (AF):
– 21 RCTs with 4201 participants: statistically significant 35% reduction, high heterogeneity.
- Coronary events:
– 10 RCTs with 77 917 participants: no statistically significant effects, no heterogeneity.
– 14 cohorts with 344 722 participants: statistically significant 11% reduction, low heterogeneity.
- Coronary deaths:
– 10 RCTs with 77 917 participants: no statistically significant effect, no heterogeneity.
– 10 cohorts with 357 621 participants: statistically significant 15% reduction, no large heterogeneity.
- Arrhythmias or sudden death:
– 12 RCTs with 43 987 participants: no statistically significant effect, low heterogeneity.
– 5 cohorts with 201 205 participants: statistically significant 47% reduction, no heterogeneity.
- CV events:
– 9 RCTs with > 72 179 participants: no statistically significant effect, low heterogeneity;
– 6 cohort studies with 68 954 participants, no statistically significant effect, large heterogeneity.
- MI:
– 20 RCTs with 86 411 participants: no statistically significant effect, low heterogeneity;
– 7 cohort studies with 274 083 participants: no statistically significant effect, large heterogeneity.
- Recurrent AF:
– 8 RCTs with 1990 participants: no statistically significant effect, high heterogeneity.
- Stroke/TIA:
– 11 RCTs with 32 026 participants: no statistically significant effect, low heterogeneity.
The other meta-analyses listed above come to similar risk reductions, although there is some inconsistency as to which CV outcomes have statistically significant results, which is most likely due to the differing inclusion criteria for RCTs, for example, some only included larger studies (more than 500 participants),108,118 whilst some only included trials that used larger doses of fish oils116 or durations of supplementation of at least 1 year.108,115,116
Dose in particular appears to be an important factor. Two meta-analyses found significant positive dose−response relationships for MI and total CVD,114 and total CVD and major vascular events,118 respectively.
A number of meta-analyses also compared lower versus higher dosages. A meta-analysis of 17 RCTs of at least 1 year duration found no significant benefits with dosages (all EPA and DHA combined) below 840 mg per day, a significantly decreased risk of cardiac death with at least 1680 mg per day (based on six RCTs), and significantly reduced risk of sudden death (based on one RCT) and stroke (based on two RCTs) with at least 2520 mg per day.115 This study found no significant benefit for all-cause mortality and non-fatal MI at any dose.
Another meta-analysis (16 RCTs with 81 073 participants) also found no significant risk reduction with a dose of 1000 mg per day, but significant reductions in cardiac mortality (9%), major CV events (10%) and MI (17%) with higher dosages.116 This study also addressed the question of whether EPA alone or in combination with DHA was more effective, with inconsistent results. Whilst the risk of cardiac death was reduced with EPA plus DHA but not EPA alone, the risk of MI and major vascular events was reduced only with EPA alone and not with the combination. It should be noted that only three of the 16 studies included in this meta-analysis actually only used EPA, all others used combinations of EPA and DHA.116
A 2020 meta-analysis of 14 RCTs (125 763 subjects, inclusion criteria: at least 500 participants, at least 1 year duration) compared low dose (≤ 1000 mg per day) versus high dose (> 1000 mg per day), and found significant risk reductions with high dose compared with control and low dose in cardiac death (21%, all versus control), MI (29%), coronary revascularisation (26%), unstable angina (27%) and major vascular events (22%).108 Risk reductions with the low dose versus control were seen in cardiac death (8%) and MI (9%), whilst no significant effects were found for all-cause mortality, stroke or sudden cardiac death.
This study made headlines as it also found a 35% increased risk of AF in the high dose compared with controls, as well as of bleeding events (49%), but not gastrointestinal disturbances.108
The issue of AF has been subject to three further meta-analyses specifically on this topic, published in 2021. One was by the same authors as the above meta-analysis, which initially reported the increased risk, and included five RCTs (the initial meta-analysis only included three RCTs for AF) and found similar results, a 37% increased risk.119 The two other meta-analyses, based on six120 and eight RCTs,121 also found statistically significantly increased risks of 31% and 51% with a high dose (more than 1000 mg per day), and 12% with a low dose (1000 mg per day or less). Except for one trial, all trials included in these meta-analyses were performed in patients with established CVD or at high risk of CVD.
A mechanism by which fish oils may increase the risk of AF is unclear,119,120 raising the question whether the association is causal.
The evidence from clinical trials suggests that EPA and DHA decrease the risk of various CV outcomes, including cardiac death and overall CV mortality, with dosages over 1000 mg per day combined EPA and DHA being more beneficial than lower dosages. At present there are insufficient data to compare EPA alone versus EPA plus DHA.
These benefits need to be weighed against the potentially increased risk of AF. It needs to be borne in mind that, whilst the relative increased risk of AF is relatively high, the absolute risk of AF is actually fairly small. For example, the REDUCE-IT trial showed significant risk reductions in various CV outcomes with a highly purified EPA product (4000 mg per day), but also found an increased risk of AF. The absolute risk of AF with EPA was 5.3% versus 3.9% with placebo, whereas the absolute risks of the primary outcomes, CV death, non-fatal MI, non-fatal stroke, coronary revascularisation and unstable angina combined were 17.2% with and 22.0% without EPA.120,122
CV risk factors
Cardiovascular disease usually develops over many years, and risk factors include high blood pressure, abnormal blood lipids, smoking and poor diet. Dyslipidaemia, in particular, has been studied extensively for benefits from fish oils.
A 2018 meta-analysis of 171 RCTs looked at the effect of fish oils on a number of CV risk factors, and found significant reductions in triglycerides (−0.368 mmol/l), systolic blood pressure (−2.195 mmHg), diastolic blood pressure (−1.08 mmHg), heart rate (−1.37 beats per minute) and CRP (−0.343 mg/l), whilst both low-density lipoprotein (LDL) and high-density lipoprotein (HDL) cholesterol increased (0.15 mmol/l and 0.039 mmol/l, respectively).123 No significant changes were found for total cholesterol, TNF-α, fibrinogen, platelet count, soluble intercellular adhesion molecule-1, soluble vascular cell adhesion molecule-1 or flow mediated dilation (a marker of endothelial function). Dosages in the reviewed studies ranged from 180 to 15 000 mg per day, and durations from 4 to 240 weeks.
A 2020 meta-analysis of 64 RCTs compared the efficacy of krill oil versus fish oil in lowering triglycerides, and found that there was no statistically significant difference between krill and fish oils.124
In some countries, including the UK, high-dose EPA EEs are licenced drugs for the treatment of hypertriglyceridaemia.125
Children: effect of maternal supplementation during pregnancy
Adequate supplies of n-3 PUFAs, especially DHA, during pregnancy are crucial for the developing foetus, and research on the effects of supplementation during pregnancy has not only focussed on maternal and neonatal outcomes (see the section ‘Pregnancy’), but also on the longer-term health of the offspring, in particular with regards to allergy and asthma, body composition and cognitive development.
Allergy/asthma
Results from observational studies suggest that intake of fish during pregnancy reduced the risk of asthma and eczema in the offspring; however, evidence from RCTs of n-3 supplementation during pregnancy give conflicting results.126
A 2019 meta-analysis of 10 RCTs including 3637 children found no significant effect of n-3 supplementation during pregnancy alone, or pregnancy and lactation/neonatal supplementation on any allergic outcomes (any allergy, eczema, asthma/wheeze, allergic rhinitis, food allergy) except sensitisation to eggs and peanuts.127 Dosages ranged from 400 to 2400 mg per day with various EPA:DHA ratios. One study used two portions of salmon and one blackcurrant seed oil rather than fish oils. There were no obvious trends with regards to particular dosages or different sources being more or less effective.
In a 2020 meta-analysis of seven RCTs including 2047 children, with follow-up periods of 6 months to 16 years, focussing on fish oil supplementation during pregnancy and asthma in the offspring only, a reduction in overall risk of asthma failed to reach statistical significance.128 However, subgroup analysis found significant reductions with a daily dose of 2000 mg combined EPA and DHA per day in those with an atopic family history and in European countries.
Overall, there is conflicting evidence for a benefit of maternal fish oils supplementation on the risk of developing childhood allergies, although those with a family history may benefit from supplementation of at least 2000 mg combined EPA and DHA per day.
Body composition/weight
Two meta-analyses investigated the potential benefits of maternal fish oil supplementation on obesity measures in the offspring. One study of 11 RCTs, including 3644 children, found no significant effects on any obesity outcomes. Dosage range was typically 400 to 1500 mg per day.129 The other included 26 RCTs with 10 970 participants, and included fish oil supplementation during pregnancy and/or lactation, with a follow up of up to 19 years.130 This study found significantly higher birth weight and postnatal waist circumference in those whose mother took fish oils compared with controls, but no significant effects on any obesity-related outcomes. The dosage range was 200−3200 mg per day, with various EPA:DHA ratios.
The current evidence suggests that maternal fish oils supplementation does not influence obesity-related outcomes in the offspring.
Cognitive development
Two meta-analyses evaluated cognitive function in the offspring of mothers who took part in fish oil supplementation trials during pregnancy.131,132 The studies included five and seven RCTs, respectively, with dosages ranging from 200 to 3300 mg per day, and neither found any association with cognitive outcomes.
Cognition
The n-3 PUFAs, and DHA in particular, are essential for the integrity and function of neuronal tissues.133 Neuronal membranes make up about 30% of brain matter and are high in DHA, which has been shown to be low in people with cognitive decline.134 Lower O3I has been associated with a more rapid cognitive decline and an earlier onset of dementia compared with a higher O3I, as reviewed by von Schacky in 2021.135
A 2020 cross-sectional study also found that higher plasma EPA and DHA levels were consistently correlated with a lower risk of dementia and cognitive decline.136 The Multidomain Alzheimer’s Preventive Trial found an O3I cut-off of 5%, below which dementia-free subjects aged 70 years or older with subjective memory complaints had increased risk of cognitive decline.137
In 2020, a meta-analysis evaluated the results of 10 clinical trials, nine of which were RCTs, and found no significant effects of DHA in preventing age-related cognitive decline.133 The DHA dose range was 190−900 mg per day, either with or without EPA, for 4−36 months. Another meta-analysis of 18 RCTs in adults without dementia found no effect on global cognitive function, but small, statistically significant improvements in memory.134 Dosage ranges were DHA 200−1550 mg per day, EPA 60−1740 mg per day, and combined 300−2800 mg per day, with durations of 4 weeks to 5 years. The heterogeneity observed in the results could not be explained by either dose or duration of supplementation.
The effect of fish oils on cognition has also been investigated in young people. A meta-analysis of 29 RCTs including 4247 people up to 25 years old found no overall effect of fish oil supplementation on cognitive function (based on standardised cognitive function tests).138 However, subgroup analysis showed significant benefits of EPA-rich, but not DHA-rich, formulations with more pronounced effects in populations with psychiatric disorders. The EPA dosage range was 0−720 mg per day, DHA dosage range 0−1200 mg per day, and combined dosage range 96−1200 mg per day, and duration was 4−48 weeks.
Overall, clinical trials do not support a role of fish oils in preventing or treating impaired cognitive function, except in young people with psychiatric disorders.
Alzheimer’s disease (AD)
There are a number of intervention studies on fish oils in AD specifically.
In 2016, a Cochrane review and meta-analysis of three RCTs, including 632 patients, concluded that EPA and DHA had no significant benefit in mild to moderate AD.139 A meta-analysis of five RCTs published in 2020 also found no effects of fish oils on cognitive function in patients with AD (no more detail available as the full article is in Spanish).140
Three studies, not included in the Cochrane review, also failed to find significant benefits for fish oils in AD, with dosages of EPA 600 mg and DHA 625 mg per day for 4 months,141 EPA 1080 mg and DHA 720 mg per day for 24 weeks,142 and EPA 1000 mg per day for 12 weeks.143
Those studies that evaluated RBC n-3 PUFAs found significant increases with supplementation.141,142,143,144 One study also found increases in DHA and EPA in the cerebrospinal fluid (CSF) following supplementation, with DHA levels in CSF being inversely related to total and phosphorylated tau protein (a marker of AD).145
Overall, at present, clinical trial data suggest that EPA and DHA are of no clinical benefit in AD.
Diabetes
Global rates of diabetes have reached epidemic proportions, and according to the World Health Organisation the number of diabetics has risen from 108 million in 1980 to 422 million in 2014.146 As diabetes is an important risk factor for CVD, the role of EPA and DHA on glycaemic control and other CV risk factors has been intensively studied.
Eight meta-analyses, including over 45 RCTs, have evaluated the potential benefits of fish oils in diabetics over the past 3 years, with most showing statistically significant improvements in some but not all markers of glycaemic control, inflammation and blood lipids, with inconsistency as to which markers were improved. Improvements have been seen in fasting blood glucose (FBG),147,148 HbA1c (glycated haemoglobin),149 fasting insulin,147 triglycerides,148,149,150 apolipoprotein A2,148 LDL cholesterol,149 TNF-α149 and IL-6.149 However, two meta-analyses did not find any benefits of n-3 PUFAs for glycaemic control in diabetics.150,151 One meta-analysis found significant benefits, in terms of reduced proteinuria, in patients with diabetic nephropathy.152
The dosage range in adults was 400−10 000 mg per day with various EPA:DHA ratios. One earlier meta-analysis carried out a subgroup analysis of EPA:DHA ratio and found ratios of > 1 to be more effective, although this failed to reach statistical significance,153 whilst none of the above studies reported any effects of dose.
One meta-analysis focussed on insulin sensitivity in children and found significant improvements. Subgroup analysis showed the best benefits with a dosage of 1500 mg per day or less, EPA:DHA higher than 1, and duration 6 months or less.154
Overall, fish oils supplementation appears to be of benefit to patients with diabetes, but there is heterogeneity between studies with regards to an effective dose, with a higher EPA:DHA ratio possibly more beneficial.
Male infertility
Infertility affects about 15% of couples worldwide, and in about half of these male infertility is present.155 Asthenozoospermia is a specific cause of male infertility characterised by reduced or absent sperm motility.156 A link between sperm motility and the DHA concentration in semen and sperm has been reported, in particular in asthenozoospermic men.156
A 2019 meta-analysis of three RCTs involving 290 infertile men reported significant increases in sperm motility and sperm DHA concentration, but no effects on sperm concentration or sperm DHA.157 Two studies used DHA only (400−800 mg per day), and the third used EPA 1120 mg and DHA 720 mg per day, with no apparent difference in results. Studies lasted for 12−32 weeks.
Three further double-blind, placebo-controlled trials have investigated the effect of DHA-rich supplements, either alone155,158 or with vitamin E,156 in infertile men, and have confirmed the positive effects on sperm motility, especially in men with asthenozoospermia. No effects on other traditional sperm or semen parameters were detected in any of the trials, except for improvements in oxidative stress in semen155 and in antioxidant status, which was associated with decreased DNA damage in the spermatozoa.158 The DHA dosage range was 465−2000 mg per day, for 10 weeks to 3 months. One study compared daily dosages of 500 mg, 1000 mg and 2000 mg of DHA, and found that improvements were seen after 1 month with 1000 mg and 2000 mg, whilst improvements with 500 mg were only seen after 3 months.155
Although there are no data on pregnancy rates, the overall evidence from clinical research suggests a benefit of DHA, either alone or with EPA, on sperm motility of infertile men, especially in men with asthenozoospermia. A dose of at least 500 mg DHA per day could be recommended.
Mental health
Depression
A review of 15 observational studies looking at n-3 levels in patients with depressive disorders found lower n-3 PUFAs, in particular DHA, compared with healthy controls, with RBC levels for DHA ranging from 0.8 to 4.0% in people with and 1.6 to 5.4% in those without depression.46 Since then, a number of further studies on O3I and depression have been published.
A study in menopausal women, taking or not taking hormone replacement therapy (HRT), evaluated the association between O3I and depression and found a small inverse association in women taking HRT, but not in those not taking HRT.159 However, all groups, with or without depression and with or without HRT, had O3I levels of > 8% on average, i.e. in the optimal range. An Australian study of 116 patients with depression and schizophrenia found average O3I levels to be 3.9%, compared with an O3I of 5% in the general Australian population, with more than half the patients having an O3I of ≤ 4% and none an O3I of ≥ 8%.160 A case−control study in patients with or without major depressive disorder (MDD) reported a mean O3I of 3.9% in those with MDD versus 5.1% in those without.161
The O3I has been shown to be lower in adolescents with or at risk of bipolar disorder (BP) compared with healthy controls, with 97% of those who had an episode of BP having an O3I ≤ 4%, compared with 61% of healthy controls.162 A case−control study in adolescents with or without depression showed DHA to be decreased in those with depression.163
In view of the overwhelming evidence that low levels of EPA and DHA are associated with depression, dozens of RCTs have been carried out evaluating the effects of EPA and/or DHA in depression.
Nine meta-analyses have been published in the last 5 years. A 2021 meta-analysis of 31 RCTs, including 41 470 participants, found no effects of n-3 PUFAs on depression or anxiety, and concluded that “Long-chain n-3 supplementation probably has little or no effect in preventing depression or anxiety symptoms”.164 However, this meta-analysis included studies with participants with and without a diagnosis of depression or anxiety, which may have weakened any effects observed in depressed patients. Another meta-analysis of four RCTs published in 2021, which looked at EPA alone or with DHA (range 1000−2000 mg combined) alongside the antidepressant drug sertraline, also found no significant effects.165
Dose appears to be an important factor. A meta-analysis of 10 RCTs comparing high (≥ 2000 mg per day combined EPA and DHA) and low dose (< 2000 mg per day) supplementation found both high and low dose to be significantly better than placebo in relieving depression in patients with MDD, and high dose to be better than low dose.166 This meta-analysis only included studies of 9 weeks or less duration, and with patients who had a diagnosis of MDD. A meta-analysis of 13 RCTs including 1233 patients with MDD also found significant benefits of fish oils in MDD.167 A subgroup analysis showed better results with higher EPA amounts (no cut-off dose reported). A 2019 meta-analysis of 26 RCTs with 2160 participants also showed significant benefits in patients with depression, especially with high-EPA formulations.168 This study showed that DHA alone or formulations with higher DHA content had no effect, whilst supplements with EPA alone or making up at least 60% and with an EPA dose of at least 1000 mg per day showed significant benefit.
Another meta-analysis of 25 RCTs involving 1373 patients with MDD also found beneficial effects of EPA and/or DHA, although the authors note that the evidence overall is of poor quality and that there was considerable heterogeneity that was not explained by subgroup or sensitivity analyses.169
Two meta-analyses looked at the potential benefits of fish oils for depression in the elderly, with conflicting results. One meta-analysis of nine RCTs found no benefit and no significant moderating effects of comorbidity, baseline depression, intervention duration and EPA:DHA ratio, potentially due to limited statistical power.170 Only three of the included RCTs were in elderly people with depression at baseline. The other meta-analysis, which included six RCTs, found benefits in people with depression (based on four studies) but not in those without depression (based on two studies).171
One meta-analysis of four RCTs looked at the potential benefits of EPA and DHA in 153 children with depression and no drug treatment, and found no significant improvements.172 Dosages ranged from 1000 mg to 3400 mg per day (combined EPA and DHA) with various EPA:DHA ratios, for 10−16 weeks.
Overall, the evidence suggests that fish oils are of benefit in adults with depression, whilst there is no effect on depressive symptoms in people without diagnosed depression. EPA appears to be of more benefit than DHA, so a pure EPA or high-EPA formula should be chosen, providing at least 1000 mg EPA.
Schizophrenia
Schizophrenia is a chronic, severe mental disorder affecting 1% of the population worldwide.173 People at high risk of schizophrenia have been found to have low O3I, with a mean of 3.0% in one study.174
A 2021 meta-analysis of 20 double-blind RCTs, involving 1494 patients with schizophrenia, found overall statistically significant improvements in psychopathology, particularly general psychopathology, and positive symptoms but not negative symptoms with fish oil supplementation.175 When EPA dose was greater than 1000 mg, there was a significant beneficial effect on general psychopathology, positive and negative symptoms, whilst with dosages of 1000 mg or less, only general psychopathology improved. Subgroup analysis showed also that benefits were greater in those with more severe disease at baseline.
Since then, two more RCTs have been published, both of which did not show any improvement in schizophrenia outcomes, but EPA dosages were below 1000 mg per day, EPA 540 mg with DHA 360 mg per day for 8 weeks,176 and EPA 360 mg with DHA 240 mg per day for 12 weeks,173 respectively, so may have been too low for positive effects. The latter study found significant improvements in brain-derived neurotrophic factor, as well as reductions in TNF-α, CRP and IL-6.173
Overall, the evidence from clinical trials suggests that fish oils with EPA dose of over 1000 mg per day are beneficial in schizophrenia.
Non-alcoholic fatty liver disease (NAFLD)
Non-alcoholic fatty liver disease is characterised by excess fat accumulation in liver cells, and is estimated to affect 20−30% of the populations in Western countries.177 Whilst low dietary n-3 PUFA intakes have been reported in patients with NAFLD, there are no epidemiological studies evaluating the O3I in this condition.178
Five meta-analyses, including over 20 RCTs altogether, have been published in the past 3 years assessing the benefits of n-3 PUFAs in NAFLD. One meta-analysis of 13 RCTs including 268 subjects looked at liver enzymes only, and found a significant decrease in alanine aminotransferase, but not other liver enzymes.179 The other four looked at a number of outcomes, one in children only,180 and all found significant decreases in liver fat content.177,178,180,181 Results for other outcomes were inconsistent; positive effects have been reported for liver enzymes,177,180,181 blood lipids177,178,181 and glycaemic control,177,181 although n-3 fats did not affect all markers (other than liver fat) in any of these studies.
Not all meta-analyses reported dosages, durations and types of n-3 fats included in their meta-analyses, and none reported any effect of dosage on outcomes through subgroup analysis.
Fish oils appear to have a number of benefits in patients with NAFLD, but there is insufficient information to make dosage recommendations.
Obesity/weight loss
In 2018, it was estimated that 41% of men in the UK were classified as overweight and 26% as obese, in women the numbers were 30% and 29%, respectively, and 1 in 5 children is classified as obese.182 Some animal models have suggested that n-3 PUFAs may play a role in weight management, but evidence in humans has been contradictory.183
A cross-sectional study from Australia found that healthy young women with obesity had a significantly lower O3I than their non-obese counterparts, 5.9% versus 6.7%, despite similar intakes.183 Another study suggests that there may be gender differences with regards to the association of O3I and obesity, with a significant inverse correlation between EPA and DHA and body mass index (BMI), waist circumference and body fat, in women but not men.184 A correlation between O3I and BMI has also been found in children, with 33% of obese children having an O3I of < 4% compared with 17% of non-obese children.185
Two meta-analyses, including 11 and 21 RCTs, respectively, have evaluated the effect of fish oils on weight loss in obesity, and both found that n-3 PUFAs can lead to a reduction in waist circumference,186,187 although in one this was only when combined with lifestyle modification (diet and/or exercise).187 Neither study found a significant effect on weight loss or BMI. Across the two meta-analyses, total n-3 dose ranged from 540 mg to 11 300 mg per day, with varying EPA:DHA ratios (EPA/DHA dosages are not reported). One of the meta-analyses reported that neither dose nor EPA:DHA ratio affected outcomes.187
One meta-analysis of six RCTs including 342 children looked at the effect of EPA and/or DHA on weight loss in children, and found no effects on either BMI, weight loss or waist circumference.188
A more recent RCT in women with depression and obesity found a beneficial effect of EPA and DHA, 1080 mg and 720 mg per day, respectively, for 12 weeks, alongside a calorie-reduced diet, with a significantly greater weight loss in the fish oil group compared with the placebo group, 3.07 kg versus 1.16 kg.189 However, at follow-up 1 month after the end of the study, women in the fish oil group had regained 2.8 kg, whilst the placebo group reduced weight by a further 0.21 kg.
Overall, the evidence suggests that fish oils may be of benefit for reducing waist circumference in adults with obesity, especially when used alongside other diet or lifestyle interventions. There are insufficient data to recommend a particular dose.
Osteoarthritis (OA)
Osteoarthritis is a common inflammatory condition of the joints, and is often considered to be due to ‘wear and tear’. Considering the high prevalence of OA (it is estimated that 9 million people in the UK have OA)190 and the popularity of fish oil supplements for this indication, clinical research on this topic is limited.
An early pilot study of 26 patients with OA found that EPA (dose reported as 10 ml) for 6 months decreased pain score by 37% and interference with activities by 30%, compared with 21% and 11%, respectively, with placebo, although the difference was not statistically significant, probably due to the small sample size.191
Since then, four more RCTs have reported benefits of fish oils, either on their own or in combination with other nutrients. A double-blind, placebo-controlled trial using EPA 400 mg and DHA 2000 mg per day for 16 weeks found significant improvements in OA-specific pain and OA burden in the fish oil group with and without curcumin, compared with curcumin alone or placebo.192 A study of glucosamine, 1500 mg per day, with or without fish oils found treatment with n-3 PUFAs, 600 mg per day (no further details) for 26 weeks, more efficacious than glucosamine alone.193
Two trials compared low- with high-dose fish oils. One of these tested EPA 400 mg and DHA 200 mg per day versus EPA 800 mg and DHA 400 mg per day versus no supplement for 8 weeks, and found significant improvements in all outcome measures with both fish oil supplements compared with control, and the low dose more beneficial than the high dose, although this was not statistically significant for all parameters.194 The other high- versus low-dose trial tested EPA and DHA 4500 mg per day (ratio 3:2) versus 450 mg per day for 2 years.195 The investigators found the low dose to lead to significantly greater improvements in pain and function than the higher dose, despite the finding that the latter increased RBC DHA and EPA more than the lower dose.
The evidence suggests that fish oils supplements are of benefit in arthritis, with lower doses possibly more effective than higher dosages. Dosages of 450−600 mg combined EPA and DHA have been shown to be effective.
Pain
Pain is closely associated with inflammation, and EPA and DHA are therefore commonly used for painful conditions other than arthritis.
Several double-blind, placebo-controlled studies have evaluated the effects of fish oils in non-arthritic musculoskeletal pain with conflicting results. A subgroup (1398 patients) of a larger trial found no benefit of EPA and DHA (combined dose 840 mg per day) on chronic knee pain over a median follow-up of 5.3 years.196 A trial of EPA 1530 mg and DHA 1040 mg per day for 8 weeks in 65 patients with rotator-cuff-related shoulder pain found significant improvements in Shoulder Pain and Disability Index after 3 months, but not in Oxford Shoulder Score.197 A study in 27 young adults found that EPA 324 mg and DHA 216 mg per day for 30 days reduced delayed-onset muscle soreness at 48 hours after eccentric training.198 And a study combining EPA 149 mg per day with serine 596 mg per day for 8 weeks has shown significant improvements in 120 patients with knee or low-back pain.199
Aromatase inhibitors (AIs) are a commonly used treatment for breast cancer, and can cause musculoskeletal pain. Two double-blind, placebo-controlled trials found no benefit of fish oils compared with placebo in AI-induced pain.200,201 The combined dosage of EPA and DHA was 4300 mg and 3300 mg per day for 24 weeks, respectively.
Randomised-controlled trials have shown promise in reducing post-operative pain in patients after weight loss surgery with EPA 3000 mg and DHA 1260 mg per day for 10 days before surgery,202 and in neonates after major heart surgery with DHA 75 mg per kg body weight per day.203
An RCT in 120 women with dysmenorrhoea (painful periods) showed that 1000 mg fish oil (no further details reported) daily for 2 months was superior to ibuprofen (taken only when needed) for pain relief.204
Another RCT compared amitriptyline alone or with DHA 250 mg and EPA 16.67 mg per day plus alpha-lipoic acid (600 mg per day), for up to 12 weeks, in 84 women with vestibulodynia (chronic pain in the vulvar area) associated with painful bladder syndrome, and found a significantly greater reduction in pain in those with the supplement compared with those on amitriptyline only.205
Overall, EPA and DHA are promising in a number of painful conditions. As both pain conditions and dosage varied widely, it is not possible to make specific recommendations, but durations of 8−12 weeks have been sufficient for more chronic types of pain, whilst in acute post-operative pain shorter durations with higher dosages were beneficial.
Polycystic ovary syndrome (PCOS)
Polycystic ovary syndrome is a common condition that affects about 1 in 10 women, and is characterised by enlarged ovaries that contain many fluid-filled sacs (follicles) that surround the eggs, excess androgens (‘male’ hormones) and irregular periods.206 Whilst generally considered an endocrine disorder, the underlying cause of PCOS is thought to be insulin resistance (IR).
Two meta-analyses have evaluated the potential benefits of fish oils in PCOS, with contradictory results. A meta-analysis of three RCTs in 2017 found no benefits with regards to IR or Homeostatic Model Assessment of Insulin Resistance (HOMA-IR).207 Dosages used in the included studies ranged from 1200 to 3600 mg n-3 PUFAs (not further described) for 6−8 weeks. A meta-analysis of nine RCTs published in 2018, on the other hand, found significant improvements in HOMA-IR (based on six studies), total and LDL cholesterol, triglycerides and adiponectin, but not sex hormone levels, BMI, fasting glucose and insulin.208 The included studies used a dosage range of 900−4000 mg total n-3 PUFAs (no further details reported) for 6−24 weeks. Neither meta-analysis included a subgroup analysis, but the positive results of the Yang et al. study were based on studies that did not appear to have much heterogeneity.
Although the evidence from the two meta-analyses is conflicting, in view of the fact that the more recent one included more studies, the overall evidence appears to be in favour of a benefit of fish oils on metabolic parameters in women with PCOS. There is insufficient information to make specific dosage recommendations.
Pregnancy
The placenta actively transports fatty acids, maintaining optimal DHA levels in the foetus of 9−10%, and an optimal O3I of 8−11% has been suggested for pregnant women.209
Two cohort studies have found a positive association between O3I and gestational length.210,211 A low O3I in late pregnancy has also been associated with an increased risk of postnatal depression.212
Gestational diabetes mellitus (GDM)
Gestational diabetes mellitus is diagnosed in about 5% of all pregnancies worldwide, and is associated with maternal and foetal complications, including macrosomia (birth weight over 4000 g) and pre-eclampsia.213,214
Three meta-analyses have evaluated the benefits of n-3 PUFAs in gestational diabetes or prediabetes, and all came to the conclusion that n-3 PUFAs can significantly improve fasting glucose, HOMA-IR and, where evaluated, high-sensitivity CRP, triglycerides and TAC, but not macrosomia, preterm delivery, neonatal hyperbilirubinaemia, NO, pre-eclampsia, gestational age, birth weight, total or LDL cholesterol.213,214,215
One meta-analysis looked at co-supplementation of n-3 PUFAs with vitamin E or vitamin D only,213 one looked at fish oils only,214 and one included studies using fish oils and flaxseed oils.215 Despite the wide range of types of supplementation, there was little heterogeneity amongst the positive results, suggesting that both marine and plant n-3 PUFAs with and without co-administration of vitamin E or vitamin D can have a benefit. Most studies used EPA 180 mg and DHA 120 mg per day for 6−8 weeks.
The clinical research suggests that in women with GDM, EPA and DHA are beneficial for glycaemic control and inflammatory status at a modest dose of EPA 180 mg and DHA 120 mg per day.
Peri-/postnatal depression
The clinical research evidence for the use of EPA and DHA in depression in general has been reviewed above. It is thought that 10−20% of pregnant women are affected by perinatal MDD.216 A meta-analysis of 12 case−control and cohort studies showed that lower serum levels of EPA, DHA and total n-3 PUFAs, and higher n-6 to n-3 ratio were associated with an increased risk of perinatal depression.217
There is a significant body of clinical research in peri- or postnatal depression, with three meta-analyses covering over 20 studies published in the last 2 years. Whilst one meta-analysis of 11 RCTs found no benefits either overall or in subgroups,218 the other two showed some benefits, although their subgroup analyses gave contradictory results. One, including 18 RCTs, found significant benefits in depressed but not non-depressed women, and in post-partum women but not during pregnancy, with the greatest effects in studies looking specifically at postnatal depression.219 The third meta-analysis found significant benefits in women with mild to moderate but not severe depression, durations of less than 8 weeks, and with EPA:DHA ratios of > 1.5.220 Dosages in all the included studies ranged from 200 to 3348 mg combined DHA and EPA or DHA only.
There appears to be a benefit of fish oil supplementation with regards to perinatal depression, although there is conflicting evidence with regards to the group most likely to benefit and the best dosages.
Preterm birth
As mentioned above, higher O3I has been associated with longer gestational period; however, evidence from intervention trials has been mixed.
A Cochrane review and meta-analysis in 2018 concluded that there was a reduced risk of preterm birth < 37 weeks (based on 26 RCTs) and early preterm birth < 34 weeks (nine RCTs) in women taking EPA and/or DHA compared with controls, based on high-quality evidence.221 This study also found that fish oils increased the risk of prolonged gestation > 42 weeks from 1.6% to 2.6%, based on six RCTs of moderate quality. The authors mention that, at the time of conducting their review, 23 clinical trials, including over 5000 women, were still ongoing.
Since then, two more meta-analyses have been published with conflicting results. A meta-analysis of 26 RCTs in 2020 found no overall decreased risk of preterm birth, but subgroup analysis showed that trials using a dose of > 1000 mg EPA plus DHA per day and a mix of EPA and DHA but not DHA alone showed benefits. The dosage range used in the included studies was 200−3120 mg per day. A meta-analysis of 37 RCTs published in 2021 found an 11% reduction in preterm birth and a 27% reduction in early preterm birth, although this lost statistical significance after sensitivity analysis.222 This meta-analysis included studies using high-omega eggs and ALA-enriched margarine as sources of n-3 PUFAs, and supplemental dosages as low as 28 mg per day (combined EPA and DHA), raising the possibility that this may have affected statistical power. No subgroup analysis was carried out.
Whilst there is conflicting evidence, EPA and DHA appear to lengthen gestational period, offering a benefit in reducing the risk of preterm birth but possibly also increasing the risk of prolonged gestation. EPA and DHA combinations appear to be more beneficial than DHA alone, and a combined dose of at least 1000 mg per day has shown benefits.
Post-traumatic stress disorder (PTSD)
Post-traumatic stress disorder is an anxiety disorder caused by very stressful, frightening or distressing events, and can have a significant impact on the person’s quality of life. It has been shown to be associated with psychophysiological symptoms, including heart rate and skin conductance, both
at rest and in response to stimuli.223
A 12-week double-blind, placebo-controlled trial found that a DHA-rich fish oil supplement (DHA 1470 mg, 147 EPA mg per day) significantly reduced heart rate at rest and after a stimulus in those on the fish oil supplement, compared with the placebo group, in serious accident survivors.223 Supplementation was started within 10 days after the traumatic event, and most participants did not develop serious psychophysiological symptoms. The mean O3I at baseline was 7.59% in the fish oil group and 7.38% in the placebo group, which increased by 3.08% in the former group.223
A small pilot study in patients with PTSD, however, was terminated early due to negative effects.224 Six patients with PTSD (trauma being 2−31 years prior to start of study) were given a high-EPA fish oil (2000 mg per day) for up to 3 months. Two patients dropped out within the first month. Of the remaining four patients, one remained unchanged and the other three had a trend towards a worsening of most symptoms and a significant worsening of the avoidance subscale of the Impact of Event Scale, raising concerns of a possible negative impact of fish oil.
The contradictory results could be due to a number of factors, including differing effects of DHA and EPA, or different treatment goals and therefore patient population (prevention versus treatment of PTSD). It is also important to note that the study reporting worsening was based on only four patients.
Until further research is carried out on the effects of fish oils on PTSD, no recommendation can be made.
Prostate cancer
A study published in 2013 raised concerns that fish oils may increase the risk of prostate cancer.225 Data from 834 men with prostate cancer were taken from a larger cohort study on the effects of vitamin E and selenium on cancer (Selenium and Vitamin E Cancer Prevention Trial, SELECT) and plasma long chain n-3 PUFAs compared with 1393 men without prostate cancer. Those in the highest quartile of plasma n-3 levels had a 43% increased risk of prostate cancer compared with those in the lowest quartile. This study has been criticised for a number of methodological problems.226
Since then, a number of meta-analyses have concluded that overall there is no association between prostate cancer and either dietary intake or biomarkers (i.e. blood levels) of n-3 PUFAs,227,228,229 except for one that showed a marginal positive association between blood DHA (but not EPA or ALA) level and prostate cancer (2% increased risk).230 One of the meta-analyses carried out a number of subgroup analyses, and found no effect of stage of prostate cancer, study design, exposure measurement or intake level on the outcomes.228
The data of these meta-analyses came mostly from epidemiological studies, over 40 altogether. Only one also looked at intervention trials, three RCTs and one uncontrolled trial.227 All four intervention trials showed no effect of supplementation on prostate-specific antigen (a marker of prostate cancer), and two trials showed a decrease in inflammatory and other cancer markers. Dosages used in the intervention trials ranged from 1600 mg to 2400 mg per day combined EPA and DHA, with three of the trials lasting for 3 months or less, and one lasting for 2 years.
Overall, the concerns raised by the results of the SELECT trial regarding an increased risk of prostate cancer with high blood levels of n-3 PUFAs have not been confirmed by a number of subsequent meta-analyses that included the SELECT trial data. At present, the evidence suggests that EPA and DHA have neither a positive nor a negative effect on prostate cancer risk.
Sleep
In animal models, n-3 PUFAs have been shown to improve sleep through a number of potential mechanisms, but evidence in humans is conflicting.231
A 2020 review and meta-analysis of 12 clinical trials and eight longitudinal studies found benefits of n-3 PUFAs on sleep architecture in infants (based on two RCTs and three cohort studies) and children with clinical sleep problems, but not in adults (seven RCTs and three cohort studies) or healthy children.231 Dosages used in the adult RCTs ranged from 220 to 2500 mg per day combined EPA and DHA, with varying ratios, including two studies that used DHA only, and one study that used salmon.
A double-blind, placebo-controlled trial in healthy adults published in 2021 found some interesting results, comparing a high-DHA (DHA 900 mg, EPA 270 mg per day) versus a high-EPA formula (EPA 900 mg, DHA 360 mg per day) versus placebo for 26 weeks.232 Participants on both the high-DHA and high-EPA formulas had improved sleep efficiency and latency, although the former only reached statistical significance in the high-DHA group. Whilst the high-EPA formula also led to decreased total sleep time and total time in bed, these increased in the DHA group compared with placebo, with the difference between the fish oil groups being statistically significant. Most interestingly though, scores for feeling rested, energetic and ready to perform decreased with DHA and increased with EPA, although only some of these differences were statistically significant.232
A double-blind, placebo-controlled trial in patients with MDD found significant effects of n-3 PUFAs (1000 mg per day, no further details) for 12 weeks on Insomnia Severity Index, with a reduction from 20.3 to 3.5 compared with 17.6 to 10.4 with placebo.233
Overall, the evidence on fish oils and sleep is conflicting, and DHA and EPA may have different effects, with EPA-rich supplements potentially being more beneficial.
Safety
A 2018 meta-analysis of 21 RCTs (24 460 participants) evaluated the safety and tolerability of prescription fish oils preparations, and found them to be generally well tolerated with no evidence of serious adverse events.234 Non-serious adverse events that were more common in the fish oil group, compared with the control group, were fishy taste and skin abnormalities (eruption, itching, exanthema or eczema), as well as abnormal laboratory values (elevated FBG, alanine transaminase and blood urea nitrogen; decreased haemoglobin and haematocrit). EPA and DHA combinations (but not EPA-only products) were also associated with belching, nausea and elevated LDL cholesterol.234
For prescription fish oil products (EPA EEs, which are licensed for hypertriglyceridaemia), the following side-effects are listed in the British National Formulary (BNF):125
Common or very common (more than 1 in 100): burping; constipation; diarrhoea; gastrointestinal discomfort; gastrointestinal disorders; nausea; vomiting.
Uncommon (1 in 1000 to 1 in 100): dizziness; gout; haemorrhage; headache; hyperglycaemia; hypotension; skin reactions; taste altered.
Rare or very rare (less than 1 in 1000): liver disorder.
As mentioned in the section ‘Cardiovascular disease’ under the heading ‘Clinical uses’, an increased risk of AF has been shown in several meta-analyses.108,119,120,121 There is also evidence for an increased risk in bleeding,108 possibly due to the inhibitory effect of fish oils on platelet aggregation. However, a meta-analysis focussing on bleeding found a reduced platelet aggregation in healthy subjects (based on 32 studies) but no increased risk of perioperative bleeding in patients undergoing surgery (based on 20 studies), leading the authors to conclude that fish oil supplements do not need to be discontinued prior to surgery.235
Animal models as well as some human studies have suggested that EPA and DHA may suppress immunity, although an increase in infections has not been seen in human studies. However, this may be relevant for people with compromised immune systems.236
The Institute of Medicine in the USA has not set an upper limit for EPA or DHA, but cautions that levels of EPA 900 mg plus DHA 600 mg per day or more for several weeks may suppress immunity, and doses of 2000–15 000 mg per day EPA and/or DHA may increase bleeding time.2 The European Food Safety Authority (EFSA) considers long-term consumption of EPA and DHA supplements at combined doses of up to about 5000 mg/day to be safe.237
Possible contamination
It is known that fish can be contaminated with dioxins, polychlorinated biphenyls (PCBs) and methylmercury.21 SCAN therefore advises pregnant and lactating women to limit their intake of fish known to be particularly high in mercury (marlin, swordfish, shark and tuna). With regards to not exceeding levels of dioxins and PCBs, women of reproductive age and girls are advised to consume 1−2 portions of oily fish per week, and women past reproductive age, boys and men 1−4 portions of oily fish per week.21
Some fish oil supplements have also been found to contain toxin levels above safe limits, including dioxins, PCBs238,239 and mercury.240 It is therefore important to choose a product from a reputable company.
Drug interactions
Anti-coagulants
Due to the effect of EPA and DHA on platelet aggregation, fish oils should only be used in combination with blood-thinning drugs when extra monitoring of coagulation status is in place.
Caution should also be exercised with any other drug that may affect bleeding.
Children
EPA and DHA supplements have been used safely in children. A meta-analysis of four RCTs, including 153 children, on the use in depression reported only one case of increased defecation, but no other adverse events from the included studies in which doses of up to 3400 mg per day EPA and DHA were used.172 A meta-analysis of 13 RCTs including 372 children with ASD reported that all adverse events were classified as mild, and were equally distributed between the fish oil and placebo groups with dosages of up to 1500 mg per day.65 Another meta-analysis of RCTs in children with ASD also reported that none of the studies found a significant difference in side-effects between placebo and omega 3 groups, and none reported serious side-effects with dosages of up to 1500 mg.63
Pregnancy/lactation
The Institute of Medicine considers intakes of 1400 mg per day n-3 PUFAs during pregnancy and 1300 mg per day during lactation as adequate.2 As for adults in general, the EFSA considers up to 5000 mg of EPA and DHA to be safe in pregnant and breastfeeding women,237 and in intervention trials dosages up to 2700 mg have been safely used during pregnancy and breastfeeding209 (see also under the section ‘Pregnancy’).
Conclusion
Although there have been some conflicting data, overall, EPA and DHA supplements have been shown to be of benefit in a wide range of disorders. Dosages and EPA:DHA ratios have varied widely in clinical trials, making it impossible to give robust dosage recommendations for most clinical uses. Not all trials have evaluated O3I at baseline, which may account for some of the discrepancies seen. Establishing O3I in clinical practice at baseline and for monitoring therapy is therefore advisable, as there is significant inter-individual variation in PUFA metabolism.
Whilst epidemiological studies have shown benefits of dietary fish intake, official recommendations of fish intake, which balance the potential benefits with the potential harm from pollutants, may be insufficient for many to achieve optimal O3I, making fish oil supplementation a valuable tool for prevention and clinical practice. Populations at risk of low O3I, such as strict vegans and vegetarians, may want to consider vegan DHA supplements.
Whilst fish oils are generally well tolerated, an increased risk of AF and bleeding has been observed in some meta-analyses, which should be borne in mind in at-risk populations.
Acknowledgements
Author contributions: K. Elgar carried out the literature review and formulated the manuscript.
Additional contributions: K. Heim with Integrative Pharmacology contributed figure 1.
Peer-reviewers and editors: the Nutritional Medicine Institute thanks the peer-reviewers and editors for their important contributions.
Funding: Open Access publication was supported by an unrestricted donation from Pure Encapsulations, Sudbury, MA, USA. No other funding or sponsorship has been received for this work.
Declaration of interest: K. Elgar has received consultancy fees from Pure Encapsulations, Sudbury, MA, USA. This article is the independent work of the author and Pure Encapsulations was not involved in the decision to publish this research.
References
1 Bang, H. O. & Dyerberg, J. (1972) Plasma lipids and lipoproteins in Greenlandic West Coast Eskimos. Acta Med. Scand., 192, 85–94.
2 IOM (2006) Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. The National Academies Press. doi:10.17226/11537.
3 Burdge, G. (2004) Alpha-linolenic acid metabolism in men and women: nutritional and biological implications. Curr. Opin. Clin. Nutr. Metab. Care, 7, 137–144.
4 Davidson, M. H. (2013) Omega-3 fatty acids: new insights into the pharmacology and biology of docosahexaenoic acid, docosapentaenoic acid, and eicosapentaenoic acid. Curr. Opin. Lipidol., 24, 467–474.
5 Imamura, S. et al. (2014) Plasma polyunsaturated fatty acid profile and delta-5 desaturase activity are altered in patients with type 2 diabetes. Metabolism, 63, 1432–1438.
6 Nicolle, L. & Hallam, A. (2010) Biochemical Imbalances in Disease. Singing Dragon.
7 Walker, R. E. et al. (2019) Predicting the effects of supplemental EPA and DHA on the omega-3 index. Am. J. Clin. Nutr., 110, 1034–1040.
8 Harris, W. S. & von Schacky, C. (2004) The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev. Med. (Baltim)., 39, 212–220.
9 von Schacky, C. (2020) Omega-3 index in 2018/19. Proc. Nutr. Soc., 11, 1–7. doi:10.1017/S0029665120006989.
10 Köhler, A., Bittner, D., Löw, A. & von Schacky, C. (2010) Effects of a convenience drink fortified with n-3 fatty acids on the n-3 index. Br. J. Nutr., 104, 729–736.
11 Simopoulos, A. P. (2011) Evolutionary aspects of diet: the omega-6/omega-3 ratio and the brain. Mol. Neurobiol., 44, 203–215.
12 Simopoulos, A. P. (2006) Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed. Pharmacother., 60, 502–507.
13 Wood, K. E., Mantzioris, E., Gibson, R. A., Ramsden, C. E. & Muhlhausler, B. S. (2015) The effect of modifying dietary LA and ALA intakes on omega-3 long chain polyunsaturated fatty acid (n-3 LCPUFA) status in human adults: a systematic review and commentary. Prostaglandins Leukot. Essent. Fatty Acids, 95, 47–55.
14 (2015) McCance and Widdowson’s The Composition of Foods. The Royal Society of Chemistry. doi:10.1039/9781849737562.
15 Corrales-Retana, L. et al. (2021) Profile of fatty acid lipid fractions of omega-3 fatty acid-enriched table eggs. J. Anim. Physiol. Anim. Nutr. (Berl.), 105, 326–335.
16 Ponnampalam, E. N., Hopkins, D. L. & Jacobs, J. L. (2018) Increasing omega-3 levels in meat from ruminants under pasture-based systems. Rev. Sci. Tech., 37, 57–70.
17 Wood, J. D. et al. (2008) Fat deposition, fatty acid composition and meat quality: A review. Meat Sci., 78, 343–358.
18 Le, H. V et al. (2018) Enhanced omega-3 polyunsaturated fatty acid contents in muscle and edible organs of Australian prime lambs grazing Lucerne and Cocksfoot pastures. Nutrients, 10, 1985.
19 Public Health England (2021) McCance and Widdowson’s composition of foods integrated dataset. Composition of foods integrated dataset (CoFID). https://www.gov.uk/government/publications/composition-of-foods-integrated-dataset-cofid.
20 Ian Givens, D. & Gibbs, R. A. (2008) Current intakes of EPA and DHA in European populations and the potential of animal-derived foods to increase them. Proc. Nutr. Soc., 67, 273–280.
21 Scientific Advisory Committee for Nutrition (SCAN) (2004) Advice on fish consumption: benefits & risks. https://www.gov.uk/government/publications/sacn-advice-on-fish-consumption.
22 Daley, C. A., Abbott, A., Doyle, P. S., Nader, G. A. & Larson, S. (2010) A review of fatty acid profiles and antioxidant content in grass-fed and grain-fed beef. Nutr. J., 9, 10.
23 McDonnell, S. L., French, C. B., Baggerly, C. A. & Harris, W. S. (2019) Cross-sectional study of the combined associations of dietary and supplemental eicosapentaenoic acid + docosahexaenoic acid on Omega-3 Index. Nutr. Res., 71, 43–55.
24 Hedengran, A., Szecsi, P. B., Dyerberg, J., Harris, W. S. & Stender, S. (2015) n-3 PUFA esterified to glycerol or as ethyl esters reduce non-fasting plasma triacylglycerol in subjects with hypertriglyceridemia: a randomized trial. Lipids, 50, 165–175.
25 Neubronner, J. et al. (2011) Enhanced increase of omega-3 index in response to long-term n-3 fatty acid supplementation from triacylglycerides versus ethyl esters. Eur. J. Clin. Nutr., 65, 247–254.
26 Lawson, L. D. & Hughes, B. G. (1988) Human absorption of fish oil fatty acids as triacylglycerols, free acids, or ethyl esters. Biochem. Biophys. Res. Commun., 152, 328–335.
27 Krokan, H. E., Bjerve, K. S. & Mørk, E. (1993) The enteral bioavailability of eicosapentaenoic acid and docosahexaenoic acid is as good from ethyl esters as from glyceryl esters in spite of lower hydrolytic rates by pancreatic lipase in vitro. Biochim. Biophys. Acta – Lipids Lipid Metab., 1168, 59–67.
28 Reis, G. J. et al. (1990) Effects of two types of fish oil supplements on serum lipids and plasma phospholipid fatty acids in coronary artery disease. Am. J. Cardiol., 66, 1171–1175.
29 Nordøy, A., Barstad, L., Connor, W. E. & Hatcher, L. (1991) Absorption of the n-3 eicosapentaenoic and docosahexaenoic acids as ethyl esters and triglycerides by humans. Am. J. Clin. Nutr., 53, 1185–1190.
30 Hansen, J. B., Olsen, J. O., Wilsgård, L., Lyngmo, V. & Svensson, B. (1993) Comparative effects of prolonged intake of highly purified fish oils as ethyl ester or triglyceride on lipids, haemostasis and platelet function in normolipaemic men. Eur. J. Clin. Nutr., 47, 497–507.
31 Dyerberg, J., Madsen, P., Møller, J. M., Aardestrup, I. & Schmidt, E. B. (2010) Bioavailability of marine n-3 fatty acid formulations. Prostaglandins Leukot. Essent. Fatty Acids, 83, 137–141.
32 Offman, E. et al. (2013) Steady-state bioavailability of prescription omega-3 on a low-fat diet is significantly improved with a free fatty acid formulation compared with an ethyl ester formulation: the ECLIPSE II study. Vasc. Health Risk Manag., 9, 563–573.
33 el Boustani, S. et al. (1987) Enteral absorption in man of eicosapentaenoic acid in different chemical forms. Lipids, 22, 711–714.
34 Hulbert, A. J., Turner, N., Storlien, L. H. & Else, P. L. (2005) Dietary fats and membrane function: implications for metabolism and disease. Biol. Rev., 80, 155–169.
35 Stillwell, W. & Wassall, S. R. (2003) Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem. Phys. Lipids, 126, 1–27.
36 Jump, D. B. (2002) The biochemistry of n-3 polyunsaturated fatty acids. J. Biol. Chem., 277, 8755–8758.
37 Calder, P. C. (2020) Eicosapentaenoic and docosahexaenoic acid derived specialised pro-resolving mediators: Concentrations in humans and the effects of age, sex, disease and increased omega-3 fatty acid intake. Biochimie, 178, 105–123.
38 Guo, X.-F., Li, K.-L., Li, J.-M. & Li, D. (2019) Effects of EPA and DHA on blood pressure and inflammatory factors: a meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr., 59, 3380–3393.
39 Morvaridzadeh, M. et al. (2020) The effects of omega-3 fatty acid supplementation on inflammatory factors in HIV-infected patients: A systematic review and meta-analysis of randomized clinical trials. Cytokine, 136, 155 298.
40 Dezfouli, M., Moeinzadeh, F., Taheri, S. & Feizi, A. (2020) The effect of omega-3 supplementation on serum levels of inflammatory biomarkers and albumin in hemodialysis patients: a systematic review and meta-analysis. J. Ren. Nutr., 30, 182–188.
41 Sepidarkish, M. et al. (2020) Effect of omega-3 fatty acid plus vitamin E co-supplementation on oxidative stress parameters: A systematic review and meta-analysis. Clin. Nutr., 39, 1019–1025.
42 Allard, J. P., Kurian, R., Aghdassi, E., Muggli, R. & Royall, D. (1997) Lipid peroxidation during n-3 fatty acid and vitamin E supplementation in humans. Lipids, 32, 535–541.
43 Harats, D. et al. (1991) Fish oil ingestion in smokers and nonsmokers enhances peroxidation of plasma lipoproteins. Atherosclerosis, 90, 127–139.
44 Heshmati, J. et al. (2019) Omega-3 fatty acids supplementation and oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Pharmacol. Res., 149, 104 462.
45 Rodríguez-Cruz, M. & Serna, D. S. (2017) Nutrigenomics of ω-3 fatty acids: Regulators of the master transcription factors. Nutrition, 41, 90–96.
46 Milte, C. M., Sinn, N. & Howe, P. R. C. (2009) Polyunsaturated fatty acid status in attention deficit hyperactivity disorder, depression, and Alzheimer’s disease: towards an omega-3 index for mental health? Nutr. Rev., 67, 573–590.
47 Crippa, A., Agostoni, C., Mauri, M., Molteni, M. & Nobile, M. (2018) Polyunsaturated fatty acids are associated with behavior but not with cognition in children with and without ADHD: an Italian study. J. Atten. Disord., 22, 971–983.
48 Chang, J. P.-C., Su, K.-P., Mondelli, V. & Pariante, C. M. (2018) Omega-3 polyunsaturated fatty acids in youths with attention deficit hyperactivity disorder: a systematic review and meta-analysis of clinical trials and biological studies. Neuropsychopharmacology, 43, 534–545.
49 Cooper, R. E., Tye, C., Kuntsi, J., Vassos, E. & Asherson, P. (2016) The effect of omega-3 polyunsaturated fatty acid supplementation on emotional dysregulation, oppositional behaviour and conduct problems in ADHD: A systematic review and meta-analysis. J. Affect. Disord., 190, 474–482.
50 Lee, J. et al. (2020) Effect of omega-3 and Korean red ginseng on children with attention deficit hyperactivity disorder: an open-label pilot study. Clin. Psychopharmacol. Neurosci., 18, 75–80.
51 Lee, J. & Lee, S. I. (2021) Efficacy of omega-3 and Korean red ginseng in children with subthreshold ADHD: a double-blind, randomized, placebo-controlled trial. J. Atten. Disord., 25, 1977−1987. doi:10.1177/1087054720951868.
52 Stoodley, I. et al. (2019) Higher omega-3 index is associated with better asthma control and lower medication dose: a cross-sectional study. Nutrients, 12, 74.
53 Hwang, I. et al. (2007) N-3 polyunsaturated fatty acids and atopy in Korean preschoolers. Lipids, 42, 345–349.
54 Woods, R. K., Thien, F. C. & Abramson, M. J. (2002) Dietary marine fatty acids (fish oil) for asthma in adults and children. Cochrane Database Syst. Rev., CD001283. doi:10.1002/14651858.CD001283.
55 Lang, J. E. et al. (2019) Fish oil supplementation in overweight/obese patients with uncontrolled asthma. A randomized trial. Ann. Am. Thorac. Soc., 16, 554–562.
56 Lee, S.-C., Yang, Y.-H., Chuang, S.-Y., Huang, S.-Y. & Pan, W.-H. (2013) Reduced medication use and improved pulmonary function with supplements containing vegetable and fruit concentrate, fish oil and probiotics in asthmatic school children: a randomised controlled trial. Br. J. Nutr., 110, 145–155.
57 Mickleborough, T. D., Lindley, M. R., Ionescu, A. A. & Fly, A. D. (2006) Protective effect of fish oil supplementation on exercise-induced bronchoconstriction in asthma. Chest, 129, 39–49.
58 Brannan, J. D. et al. (2015) The effect of omega-3 fatty acids on bronchial hyperresponsiveness, sputum eosinophilia, and mast cell mediators in asthma. Chest, 147, 397–405.
59 Abdo-Sultan, M. K., Abd-El-Lateef, R. S. & Kamel, F. Z. (2019) Efficacy of omega-3 fatty acids supplementation versus sublingual immunotherapy in patients with bronchial asthma. Egypt. J. Immunol., 26, 79–89.
60 Farjadian, S., Moghtaderi, M., Kalani, M., Gholami, T. & Hosseini Teshnizi, S. (2016) Effects of omega-3 fatty acids on serum levels of T-helper cytokines in children with asthma. Cytokine, 85, 61–66.
61 Bjørneboe, A., Søyland, E., Bjørneboe, G. E., Rajka, G. & Drevon, C. A. (1987) Effect of dietary supplementation with eicosapentaenoic acid in the treatment of atopic dermatitis. Br. J. Dermatol., 117, 463–469.
62 Koch, C. et al. (2008) Docosahexaenoic acid (DHA) supplementation in atopic eczema: a randomized, double-blind, controlled trial. Br. J. Dermatol., 158, 786–792.
63 Cheng, Y.-S. et al. (2017) Supplementation of omega 3 fatty acids may improve hyperactivity, lethargy, and stereotypy in children with autism spectrum disorders: a meta-analysis of randomized controlled trials. Neuropsychiatr. Dis. Treat., 13, 2531–2543.
64 Mazahery, H. et al. (2019) A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. J. Steroid Biochem. Mol. Biol., 187, 9–16.
65 de Andrade Wobido, K. et al. (2021) Non-specific effect of omega-3 fatty acid supplementation on autistic spectrum disorder: systematic review and meta-analysis. Nutr. Neurosci., 1–13. doi:10.1080/1028415X.2021.1913950.
66 Horvath, A., Łukasik, J. & Szajewska, H. (2017) ω-3 Fatty acid supplementation does not affect autism spectrum disorder in children: a systematic review and meta-analysis. J. Nutr., 147, 367–376.
67 Doaei, S. et al. (2021) The effect of omega-3 fatty acids supplementation on social and behavioral disorders of children with autism: a randomized clinical trial. Pediatr. Endocrinol. Diabetes. Metab., 27, 12–18.
68 Brennan Laing, B., Cavadino, A., Ellett, S. & Ferguson, L. R. (2020) Effects of an omega-3 and vitamin D supplement on fatty acids and vitamin D serum levels in double-blinded, randomized, controlled trials in healthy and Crohn’s disease populations. Nutrients, 12, 1139.
69 Feagan, B. G. et al. (2008) Omega-3 free fatty acids for the maintenance of remission in Crohn disease: the EPIC Randomized Controlled Trials. JAMA, 299, 1690–1697.
70 Romano, C., Cucchiara, S., Barabino, A., Annese, V. & Sferlazzas, C. (2005) Usefulness of omega-3 fatty acid supplementation in addition to mesalazine in maintaining remission in pediatric Crohn’s disease: a double-blind, randomized, placebo-controlled study. World J. Gastroenterol., 11, 7118–7121.
71 Nielsen, A. A. et al. (2005) Omega-3 fatty acids inhibit an increase of proinflammatory cytokines in patients with active Crohn’s disease compared with omega-6 fatty acids. Aliment. Pharmacol. Ther., 22, 1121–1128.
72 Scaioli, E. et al. (2018) Eicosapentaenoic acid reduces fecal levels of calprotectin and prevents relapse in patients with ulcerative colitis. Clin. Gastroenterol. Hepatol., 16, 1268-1275.e2.
73 Stenson, W. F. et al. (1992) Dietary supplementation with fish oil in ulcerative colitis. Ann. Intern. Med., 116, 609–614.
74 Aslan, A. & Triadafilopoulos, G. (1992) Fish oil fatty acid supplementation in active ulcerative colitis: a double-blind, placebo-controlled, crossover study. Am. J. Gastroenterol., 87, 432–437.
75 Loeschke, K. et al. (1996) N-3 fatty acids only delay early relapse of ulcerative colitis in remission. Dig. Dis. Sci., 41, 2087–2094.
76 Greenfield, S. M. et al. (1993) A randomized controlled study of evening primrose oil and fish oil in ulcerative colitis. Aliment. Pharmacol. Ther., 7, 159–166.
77 Barbosa, D. S. et al. (2003) Decreased oxidative stress in patients with ulcerative colitis supplemented with fish oil omega-3 fatty acids. Nutrition, 19, 837–842.
78 Shimizu, T. et al. (2003) Effects of highly purified eicosapentaenoic acid on erythrocyte fatty acid composition and leukocyte and colonic mucosa leukotriene B4 production in children with ulcerative colitis. J. Pediatr. Gastroenterol. Nutr., 37, 581–585.
79 Hawthorne, A. B. et al. (1992) Treatment of ulcerative colitis with fish oil supplementation: a prospective 12 month randomised controlled trial. Gut, 33, 922–928.
80 Salomon, P., Kornbluth, A. A. & Janowitz, H. D. (1990) Treatment of ulcerative colitis with fish oil n-3-omega-fatty acid: an open trial. J. Clin. Gastroenterol., 12, 157–161.
81 McCall, T. B., O’Leary, D., Bloomfield, J. & O’Moráin, C. A. (1989) Therapeutic potential of fish oil in the treatment of ulcerative colitis. Aliment. Pharmacol. Ther., 3, 415–424.
82 Prossomariti, A. et al. (2017) Short-term treatment with eicosapentaenoic acid improves inflammation and affects colonic differentiation markers and microbiota in patients with ulcerative colitis. Sci. Rep., 7, 7458.
83 Ramirez-Ramirez, V. et al. (2013) Efficacy of fish oil on serum of TNFα, IL-1β, and IL-6 oxidative stress markers in multiple sclerosis treated with interferon beta-1b. Oxid. Med. Cell. Longev., 2013, 709 493.
84 Sedighiyan, M., Djafarian, K., Dabiri, S., Abdolahi, M. & Shab-Bidar, S. (2019) The effects of omega-3 supplementation on the Expanded Disability Status Scale and inflammatory cytokines in multiple sclerosis patients: a systematic review and meta-analysis. CNS Neurol. Disord. Drug Targets, 18, 523–529.
85 Zandi-Esfahan, S. et al. (2017) Evaluating the effect of adding fish oil to Fingolimod on TNF-α, IL1β, IL6, and IFN-γ in patients with relapsing-remitting multiple sclerosis: A double-blind randomized placebo-controlled trial. Clin. Neurol. Neurosurg., 163, 173–178.
86 Sorto-Gomez, T. E. et al. (2016) Effect of fish oil on glutathione redox system in multiple sclerosis. Am. J. Neurodegener. Dis., 5, 145–151.
87 Shinto, L. et al. (2009) Omega-3 fatty acid supplementation decreases matrix metalloproteinase-9 production in relapsing-remitting multiple sclerosis. Prostaglandins Leukot. Essent. Fatty Acids, 80, 131–136.
88 Clark, C. C. T., Taghizadeh, M., Nahavandi, M. & Jafarnejad, S. (2019) Efficacy of ω-3 supplementation in patients with psoriasis: a meta-analysis of randomized controlled trials. Clin. Rheumatol., 38, 977–988.
89 Yang, S.-J. & Chi, C.-C. (2019) Effects of fish oil supplement on psoriasis: a meta-analysis of randomized controlled trials. BMC Complement. Altern. Med., 19, 354.
90 Gioxari, A., Kaliora, A. C., Marantidou, F. & Panagiotakos, D. P. (2018) Intake of ω-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: A systematic review and meta-analysis. Nutrition, 45, 114−124.e4.
91 Nordström, D. C. E. et al. (1995) Alpha-linolenic acid in the treatment of rheumatoid arthritis. A double-blind, placebo-controlled and randomized study: flaxseed vs. safflower seed. Rheumatol. Int., 14, 231–234.
92 Rajaei, E. et al. (2015) The effect of omega-3 fatty acids in patients with active rheumatoid arthritis receiving DMARDs therapy: double-blind randomized controlled trial. Glob. J. Health Sci., 8, 18–25.
93 Proudman, S. M. et al. (2015) Fish oil in recent onset rheumatoid arthritis: a randomised, double-blind controlled trial within algorithm-based drug use. Ann. Rheum. Dis., 74, 89–95.
94 Kremer, J. M. et al. (1995) Effects of high-dose fish oil on rheumatoid arthritis after stopping nonsteroidal antiinflammatory drugs. Clinical and immune correlates. Arthritis Rheum., 38, 1107–1114.
95 Lau, C. S., Morley, K. D. & Belch, J. J. (1993) Effects of fish oil supplementation on non-steroidal anti-inflammatory drug requirement in patients with mild rheumatoid arthritis-a double-blind placebo controlled study. Br. J. Rheumatol., 32, 982–989.
96 Kjeldsen-Kragh, J. et al. (1992) Dietary omega-3 fatty acid supplementation and naproxen treatment in patients with rheumatoid arthritis. J. Rheumatol., 19, 1531–1536.
97 Sköldstam, L., Börjesson, O., Kjällman, A., Seiving, B. & Akesson, B. (1992) Effect of six months of fish oil supplementation in stable rheumatoid arthritis. A double-blind, controlled study. Scand. J. Rheumatol., 21, 178–185.
98 Kremer, J. M. et al. (1987) Fish-oil fatty acid supplementation in active rheumatoid arthritis. A double-blinded, controlled, crossover study. Ann. Intern. Med., 106, 497–503.
99 Geusens, P., Wouters, C., Nijs, J., Jiang, Y. & Dequeker, J. (1994) Long-term effect of omega-3 fatty acid supplementation in active rheumatoid arthritis. A 12-month, double-blind, controlled study. Arthritis Rheum., 37, 824–829.
100 Gheita, T., Kamel, S., Helmy, N., El-Laithy, N. & Monir, A. (2012) Omega-3 fatty acids in juvenile idiopathic arthritis: effect on cytokines (IL-1 and TNF-α), disease activity and response criteria. Clin. Rheumatol., 31, 363–366.
101 Dawczynski, C. et al. (2018) Docosahexaenoic acid in the treatment of rheumatoid arthritis: A double-blind, placebo-controlled, randomized cross-over study with microalgae vs. sunflower oil. Clin. Nutr., 37, 494–504.
102 Lozovoy, M. A. B. et al. (2015) Fish oil N-3 fatty acids increase adiponectin and decrease leptin levels in patients with systemic lupus erythematosus. Mar. Drugs, 13, 1071–1083.
103 Aghdassi, E. et al. (2011) Alterations in circulating fatty acid composition in patients with systemic lupus erythematosus: a pilot study. JPEN J. Parenter. Enteral Nutr., 35, 198–208.
104 Duarte-García, A. et al. (2020) Effect of omega-3 fatty acids on systemic lupus erythematosus disease activity: A systematic review and meta-analysis. Autoimmun. Rev., 19, 102 688.
105 Walton, A. J. et al. (1991) Dietary fish oil and the severity of symptoms in patients with systemic lupus erythematosus. Ann. Rheum. Dis., 50, 463–466.
106 Westberg, G. & Tarkowski, A. (1990) Effect of MaxEPA in patients with SLE. A double-blind, crossover study. Scand. J. Rheumatol., 19, 137–143.
107 Clark, W. F. et al. (1993) Fish oil in lupus nephritis: clinical findings and methodological implications. Kidney Int., 44, 75–86.
108 Lombardi, M. et al. (2020) Impact of different doses of omega-3 fatty acids on cardiovascular outcomes: a pairwise and network meta-analysis. Curr. Atheroscler. Rep., 22, 45.
109 Harris, W. S. (2008) The omega-3 index as a risk factor for coronary heart disease. Am. J. Clin. Nutr., 87, 1997S−2002S.
110 Harris, W. S., Del Gobbo, L. & Tintle, N. L. (2017) The Omega-3 Index and relative risk for coronary heart disease mortality: Estimation from 10 cohort studies. Atherosclerosis, 262, 51–54.
111 Harris, W. S., Tintle, N. L., Etherton, M. R. & Vasan, R. S. (2018) Erythrocyte long-chain omega-3 fatty acid levels are inversely associated with mortality and with incident cardiovascular disease: The Framingham Heart Study. J. Clin. Lipidol., 12, 718−727.e6.
112 Aung, T. et al. (2018) Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77 917 individuals. JAMA Cardiol., 3, 225–234.
113 Choi, H. et al. (2021) Omega-3 fatty acids supplementation on major cardiovascular outcomes: an umbrella review of meta-analyses of observational studies and randomized controlled trials. Eur. Rev. Med. Pharmacol. Sci., 25, 2079–2092.
114 Bernasconi, A. A., Wiest, M. M., Lavie, C. J., Milani, R. V. & Laukkanen, J. A. (2021) Effect of omega-3 dosage on cardiovascular outcomes: an updated meta-analysis and meta-regression of interventional trials. Mayo Clin. Proc., 96, 304–313.
115 Rizos, E. C., Markozannes, G., Tsapas, A., Mantzoros, C. S. & Ntzani, E. E. (2021) Omega-3 supplementation and cardiovascular disease: formulation-based systematic review and meta-analysis with trial sequential analysis. Heart, 107, 150–158.
116 Casula, M. et al. (2020) Omega-3 polyunsaturated fatty acids supplementation and cardiovascular outcomes: do formulation, dosage, and baseline cardiovascular risk matter? An updated meta-analysis of randomized controlled trials. Pharmacol. Res., 160, 105 060.
117 Cabiddu, M. F., Russi, A., Appolloni, L., Mengato, D. & Chiumente, M. (2020) Omega-3 for the prevention of cardiovascular diseases: meta-analysis and trial-sequential analysis. Eur. J. Hosp. Pharm. Sci. Pract. doi:10.1136/ejhpharm-2020-002207.
118 Hu, Y., Hu, F. B. & Manson, J. E. (2019) Marine omega-3 supplementation and cardiovascular disease: an updated meta-analysis of 13 randomized controlled trials involving 127 477 participants. J. Am. Heart Assoc., 8, e013543.
119 Lombardi, M. et al. (2021) Omega-3 fatty acids supplementation and risk of atrial fibrillation: an updated meta-analysis of randomized controlled trials. Eur. Heart J. Cardiovasc. Pharmacother., 7, e69−e70. doi:10.1093/ehjcvp/pvab008.
120 Kow, C. S., Doi, S. A. R. & Hasan, S. S. (2021) The coincidence of increased risk of atrial fibrillation in randomized control trials of omega-3 fatty acids: a meta-analysis. Expert Rev. Clin. Pharmacol., 1–3. doi:10.1080/17512433.2021.1913051.
121 Jia, X. et al. (2021) Association between omega-3 fatty acid treatment and atrial fibrillation in cardiovascular outcome trials: a systematic review and meta-analysis. Cardiovasc. Drugs Ther., 35, 793−800. doi:10.1007/s10557-021-07204-z.
122 Bhatt, D. L. et al. (2018) Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N. Engl. J. Med., 380, 11–22.
123 AbuMweis, S., Jew, S., Tayyem, R. & Agraib, L. (2018) Eicosapentaenoic acid and docosahexaenoic acid containing supplements modulate risk factors for cardiovascular disease: a meta-analysis of randomised placebo-control human clinical trials. J. Hum. Nutr. Diet., 31, 67–84.
124 Kim, M. G., Yang, I., Lee, H. S., Lee, J.-Y. & Kim, K. (2020) Lipid-modifying effects of krill oil vs fish oil: a network meta-analysis. Nutr. Rev., 78, 699–708.
125 National Insitute for Health and Care Excellence (NICE). Omega-3-acid ethyl esters. British National Formulary (BNF). https://bnf.nice.org.uk/drug/omega-3-acid-ethyl-esters.html.
126 Best, K. P., Gold, M., Kennedy, D., Martin, J. & Makrides, M. (2016) Omega-3 long-chain PUFA intake during pregnancy and allergic disease outcomes in the offspring: a systematic review and meta-analysis of observational studies and randomized controlled trials. Am. J. Clin. Nutr., 103, 128–143.
127 Vahdaninia, M., Mackenzie, H., Dean, T. & Helps, S. (2019) ω-3 LCPUFA supplementation during pregnancy and risk of allergic outcomes or sensitization in offspring: A systematic review and meta-analysis. Ann. Allergy Asthma Immunol., 122, 302−313.e2.
128 Lin, J., Zhang, Y., Zhu, X., Wang, D. & Dai, J. (2020) Effects of supplementation with omega-3 fatty acids during pregnancy on asthma or wheeze of children: a systematic review and meta-analysis. J. Matern. Fetal. Neonatal Med., 33, 1792–1801.
129 Vahdaninia, M., Mackenzie, H., Dean, T. & Helps, S. (2019) The effectiveness of ω-3 polyunsaturated fatty acid interventions during pregnancy on obesity measures in the offspring: an up-to-date systematic review and meta-analysis. Eur. J. Nutr., 58, 2597–2613.
130 Li, G.-L., Chen, H.-J., Zhang, W.-X., Tong, Q. & Yan, Y.-E. (2018) Effects of maternal omega-3 fatty acids supplementation during pregnancy/lactation on body composition of the offspring: A systematic review and meta-analysis. Clin. Nutr., 37, 1462–1473.
131 Gould, J. F., Smithers, L. G. & Makrides, M. (2013) The effect of maternal omega-3 (n-3) LCPUFA supplementation during pregnancy on early childhood cognitive and visual development: a systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr., 97, 531–544.
132 Lehner, A. et al. (2021) Impact of omega-3 fatty acid DHA and EPA supplementation in pregnant or breast-feeding women on cognitive performance of children: systematic review and meta-analysis. Nutr. Rev., 79, 585–598.
133 Balachandar, R., Soundararajan, S. & Bagepally, B. S. (2020) Docosahexaenoic acid supplementation in age-related cognitive decline: a systematic review and meta-analysis. Eur. J. Clin. Pharmacol., 76, 639–648.
134 Alex, A., Abbott, K. A., McEvoy, M., Schofield, P. W. & Garg, M. L. (2020) Long-chain omega-3 polyunsaturated fatty acids and cognitive decline in non-demented adults: a systematic review and meta-analysis. Nutr. Rev., 78, 563–578.
135 von Schacky, C. (2021) Importance of EPA and DHA blood levels in brain structure and function. Nutrients, 13, 1074.
136 Thomas, A. et al. (2020) Blood polyunsaturated omega-3 fatty acids, brain atrophy, cognitive decline, and dementia risk. Alzheimers Dement., doi:10.1002/alz.12195.
137 Coley, N. et al. (2018) Defining the optimal target population for trials of polyunsaturated fatty acid supplementation using the erythrocyte Omega-3 Index: a step towards personalized prevention of cognitive decline? J. Nutr. Health Aging, 22, 982–998.
138 Emery, S. et al. (2020) Omega-3 and its domain-specific effects on cognitive test performance in youths: A meta-analysis. Neurosci. Biobehav. Rev., 112, 420–436.
139 Burckhardt, M. et al. (2016) Omega-3 fatty acids for the treatment of dementia. Cochrane Database Syst. Rev., 4, CD009002.
140 Araya-Quintanilla, F. et al. (2020) Effectiveness of omega-3 fatty acid supplementation in patients with Alzheimer disease: A systematic review and meta-analysis. Neurologia, 35, 105–114.
141 Phillips, M. A., Childs, C. E., Calder, P. C. & Rogers, P. J. (2015) No effect of omega-3 fatty acid supplementation on cognition and mood in individuals with cognitive impairment and probable Alzheimer’s disease: a randomised controlled trial. Int. J. Mol. Sci., 16, 24 600–24 613.
142 Chiu, C.-C. et al. (2008) The effects of omega-3 fatty acids monotherapy in Alzheimer’s disease and mild cognitive impairment: a preliminary randomized double-blind placebo-controlled study. Prog. Neuropsychopharmacol. Biol. Psychiatry, 32, 1538–1544.
143 Boston, P. F., Bennett, A., Horrobin, D. F. & Bennett, C. N. (2004) Ethyl-EPA in Alzheimer’s disease-a pilot study. Prostaglandins Leukot. Essent. Fatty Acids, 71, 341–346.
144 Eriksdotter, M. et al. (2015) Plasma fatty acid profiles in relation to cognition and gender in Alzheimer’s disease patients during oral omega-3 fatty acid supplementation: the OmegAD Study. J. Alzheimers Dis., 48, 805–812.
145 Freund Levi, Y. et al. (2014) Transfer of omega-3 fatty acids across the blood-brain barrier after dietary supplementation with a docosahexaenoic acid-rich omega-3 fatty acid preparation in patients with Alzheimer’s disease: the OmegAD study. J. Intern. Med., 275, 428–436.
146 Becic, T. & Studenik, C. (2018) Effects of omega-3 supplementation on adipocytokines in prediabetes and type 2 diabetes mellitus: systematic review and meta-analysis of randomized controlled trials. Diabetes Metab. J., 42, 101–116.
147 Delpino, F. M. et al. (2021) Omega-3 supplementation and diabetes: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr., 1–14. doi:10.1080/10408398.2021.1875977.
148 Natto, Z. S., Yaghmoor, W., Alshaeri, H. K. & Van Dyke, T. E. (2019) Omega-3 fatty acids effects on inflammatory biomarkers and lipid profiles among diabetic and cardiovascular disease patients: a systematic review and meta-analysis. Sci. Rep., 9, 18 867.
149 O’Mahoney, L. L. et al. (2018) Omega-3 polyunsaturated fatty acids favourably modulate cardiometabolic biomarkers in type 2 diabetes: a meta-analysis and meta-regression of randomized controlled trials. Cardiovasc. Diabetol., 17, 98.
150 Gao, C. et al. (2020) Effects of fish oil supplementation on glucose control and lipid levels among patients with type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Lipids Health Dis., 19, 87.
151 Brown, T. J. et al. (2019) Omega-3, omega-6, and total dietary polyunsaturated fat for prevention and treatment of type 2 diabetes mellitus: systematic review and meta-analysis of randomised controlled trials. BMJ, 366, l4 697.
152 Chewcharat, A., Chewcharat, P., Rutirapong, A. & Papatheodorou, S. (2020) The effects of omega-3 fatty acids on diabetic nephropathy: A meta-analysis of randomized controlled trials. PLoS One, 15, e0228315.
153 Chen, C., Yu, X. & Shao, S. (2015) Effects of omega-3 fatty acid supplementation on glucose control and lipid levels in type 2 diabetes: a meta-analysis. PLoS One, 10, e0139565.
154 Hou, M. et al. (2021) Effect of fish oil on insulin sensitivity in children: a systematic review and meta-analysis of randomized, controlled trials. Can. J. Diabetes, 45, 531−538.e1. doi:10.1016/j.jcjd.2020.11.004.
155 González-Ravina, C. et al. (2018) Effect of dietary supplementation with a highly pure and concentrated docosahexaenoic acid (DHA) supplement on human sperm function. Reprod. Biol., 18, 282–288.
156 Eslamian, G., Amirjannati, N., Noori, N., Sadeghi, M.-R. & Hekmatdoost, A. (2020) Effects of coadministration of DHA and vitamin E on spermatogram, seminal oxidative stress, and sperm phospholipids in asthenozoospermic men: a randomized controlled trial. Am. J. Clin. Nutr., 112, 707–719.
157 Hosseini, B. et al. (2019) The effect of omega-3 fatty acids, EPA, and/or DHA on male infertility: a systematic review and meta-analysis. J. Diet. Suppl., 16, 245–256.
158 Martínez-Soto, J. C. et al. (2016) Dietary supplementation with docosahexaenoic acid (DHA) improves seminal antioxidant status and decreases sperm DNA fragmentation. Syst. Biol. Reprod. Med., 62, 387–395.
159 Jin, Y., Kim, T.-H. & Park, Y. (2016) Association between erythrocyte levels of n-3 polyunsaturated fatty acids and depression in postmenopausal women using or not using hormone therapy. Menopause, 23, 1012–1018.
160 Parletta, N. et al. (2016) People with schizophrenia and depression have a low omega-3 index. Prostaglandins Leukot. Essent. Fatty Acids, 110, 42–47.
161 Baghai, T. C. et al. (2011) Major depressive disorder is associated with cardiovascular risk factors and low Omega-3 Index. J. Clin. Psychiatry, 72, 1242–1247.
162 McNamara, R. K. et al. (2016) Adolescents with or at ultra-high risk for bipolar disorder exhibit erythrocyte docosahexaenoic acid and eicosapentaenoic acid deficits: a candidate prodromal risk biomarker. Early Interv. Psychiatry, 10, 203–211.
163 Pottala, J. V et al. (2012) Red blood cell fatty acids are associated with depression in a case-control study of adolescents. Prostaglandins Leukot. Essent. Fatty Acids, 86, 161–165.
164 Deane, K. H. O. et al. (2021) Omega-3 and polyunsaturated fat for prevention of depression and anxiety symptoms: systematic review and meta-analysis of randomised trials. Br. J. Psychiatry, 218, 135–142.
165 Chambergo-Michilot, D., Brañez-Condorena, A., Falvy-Bockos, I., Pacheco-Mendoza, J. & Benites-Zapata, V. A. (2021) Efficacy of omega-3 supplementation on sertraline continuous therapy to reduce depression or anxiety symptoms: A systematic review and meta-analysis. Psychiatry Res., 296, 113 652.
166 Luo, X.-D. et al. (2020) High-dose omega-3 polyunsaturated fatty acid supplementation might be more superior than low-dose for major depressive disorder in early therapy period: a network meta-analysis. BMC Psychiatry, 20, 248.
167 Mocking, R. J. T. et al. (2016) Meta-analysis and meta-regression of omega-3 polyunsaturated fatty acid supplementation for major depressive disorder. Transl. Psychiatry, 6, e756.
168 Liao, Y. et al. (2019) Efficacy of omega-3 PUFAs in depression: A meta-analysis. Transl. Psychiatry, 9, 190.
169 Appleton, K. M., Sallis, H. M., Perry, R., Ness, A. R. & Churchill, R. (2016) ω-3 Fatty acids for major depressive disorder in adults: an abridged Cochrane review. BMJ Open, 6, e010172.
170 Bai, Z.-G., Bo, A., Wu, S.-J., Gai, Q.-Y. & Chi, I. (2018) Omega-3 polyunsaturated fatty acids and reduction of depressive symptoms in older adults: A systematic review and meta-analysis. J. Affect. Disord., 241, 241–248.
171 Bae, J.-H. & Kim, G. (2018) Systematic review and meta-analysis of omega-3-fatty acids in elderly patients with depression. Nutr. Res., 50, 1–9.
172 Zhang, L., Liu, H., Kuang, L., Meng, H. & Zhou, X. (2019) Omega-3 fatty acids for the treatment of depressive disorders in children and adolescents: a meta-analysis of randomized placebo-controlled trials. Child Adolesc. Psychiatry Ment. Health, 13, 36.
173 Tang, W. et al. (2020) Omega-3 fatty acids ameliorate cognitive dysfunction in schizophrenia patients with metabolic syndrome. Brain. Behav. Immun., 88, 529–534.
174 Cadenhead, K. S. et al. (2019) Metabolic abnormalities and low dietary Omega 3 are associated with symptom severity and worse functioning prior to the onset of psychosis: Findings from the North American Prodrome Longitudinal Studies Consortium. Schizophr. Res., 204, 96–103.
175 Goh, K. K., Chen, C. Y.-A., Chen, C.-H. & Lu, M.-L. (2021) Effects of omega-3 polyunsaturated fatty acids supplements on psychopathology and metabolic parameters in schizophrenia: A meta-analysis of randomized controlled trials. J. Psychopharmacol., 35, 221–235.
176 Qiao, Y. et al. (2020) No impact of omega-3 fatty acid supplementation on symptoms or hostility among patients with schizophrenia. Front. Psychiatry, 11, 312.
177 Musa-Veloso, K. et al. (2018) Systematic review and meta-analysis of controlled intervention studies on the effectiveness of long-chain omega-3 fatty acids in patients with nonalcoholic fatty liver disease. Nutr. Rev., 76, 581–602.
178 Lee, C.-H., Fu, Y., Yang, S.-J. & Chi, C.-C. (2020) Effects of omega-3 polyunsaturated fatty acid supplementation on non-alcoholic fatty liver: a systematic review and meta-analysis. Nutrients, 12, 2769.
179 Yu, L., Yuan, M. & Wang, L. (2017) The effect of omega-3 unsaturated fatty acids on non-alcoholic fatty liver disease: A systematic review and meta-analysis of RCTs. Pakistan J. Med. Sci., 33, 1022–1028.
180 Chen, L.-H., Wang, Y.-F., Xu, Q.-H. & Chen, S.-S. (2018) Omega-3 fatty acids as a treatment for non-alcoholic fatty liver disease in children: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr., 37, 516–521.
181 Yan, J.-H., Guan, B.-J., Gao, H.-Y. & Peng, X.-E. (2018) Omega-3 polyunsaturated fatty acid supplementation and non-alcoholic fatty liver disease: A meta-analysis of randomized controlled trials. Medicine (Baltimore), 97, e12271.
182 Lifestyles Team NHS Digital (2020) Statistics on Obesity, Physical Activity and Diet, England, 2020. https://digital.nhs.uk/data-and-information/publications/statistical/statistics-on-obesity-physical-activity-and-diet/england-2020.
183 Young, I. E. et al. (2020) Association between obesity and omega-3 status in healthy young women. Nutrients, 12, 1480.
184 Howe, P. R. C. et al. (2014) Relationship between erythrocyte omega-3 content and obesity is gender dependent. Nutrients, 6, 1850–1860.
185 Burrows, T., Collins, C. E. & Garg, M. L. (2011) Omega-3 index, obesity and insulin resistance in children. Int. J. Pediatr. Obes., 6, e532−e539.
186 Zhang, Y. Y., Liu, W., Zhao, T. Y. & Tian, H. M. (2017) Efficacy of omega-3 polyunsaturated fatty acids supplementation in managing overweight and obesity: a meta-analysis of randomized clinical trials. J. Nutr. Health Aging, 21, 187–192.
187 Du, S., Jin, J., Fang, W. & Su, Q. (2015) Does fish oil have an anti-obesity effect in overweight/obese adults? A meta-analysis of randomized controlled trials. PLoS One, 10, e0142652.
188 Jazayeri, S. et al. (2020) Effect of omega-3 fatty acids supplementation on anthropometric indices in children and adolescents: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med., 53, 102 487.
189 Keshavarz, S. A. et al. (2018) Omega-3 supplementation effects on body weight and depression among dieter women with co-morbidity of depression and obesity compared with the placebo: A randomized clinical trial. Clin. Nutr. ESPEN, 25, 37–43.
190 National Insitute for Health and Care Excellence (NICE) (2018) Osteoarthritis. NICE health topics. https://cks.nice.org.uk/topics/osteoarthritis/.
191 Stammers, T., Sibbald, B. & Freeling, P. (1989) Fish oil in osteoarthritis. Lancet (London, England), 2, 503.
192 Kuszewski, J. C., Wong, R. H. X. & Howe, P. R. C. (2020) Fish oil supplementation reduces osteoarthritis-specific pain in older adults with overweight/obesity. Rheumatol. Adv. Pract., 4, rkaa036.
193 Gruenwald, J., Petzold, E., Busch, R., Petzold, H.-P. & Graubaum, H.-J. (2009) Effect of glucosamine sulfate with or without omega-3 fatty acids in patients with osteoarthritis. Adv. Ther., 26, 858–871.
194 Peanpadungrat, P. (2015) Efficacy and safety of fish oil in treatment of knee osteoarthritis. J. Med. Assoc. Thai., 98 Suppl 3, S110−S114.
195 Hill, C. L. et al. (2016) Fish oil in knee osteoarthritis: a randomised clinical trial of low dose versus high dose. Ann. Rheum. Dis., 75, 23–29.
196 MacFarlane, L. A. et al. (2020) The effects of vitamin D and marine omega-3 fatty acid supplementation on chronic knee pain in older US adults: results from a randomized trial. Arthritis Rheumatol. (Hoboken, N.J.), 72, 1836–1844.
197 Sandford, F. M., Sanders, T. A., Wilson, H. & Lewis, J. S. (2018) A randomised controlled trial of long-chain omega-3 polyunsaturated fatty acids in the management of rotator cuff related shoulder pain. BMJ Open Sport Exerc. Med., 4, e000414.
198 Tartibian, B., Maleki, B. H. & Abbasi, A. (2009) The effects of ingestion of omega-3 fatty acids on perceived pain and external symptoms of delayed onset muscle soreness in untrained men. Clin. J. Sport Med., 19, 115–119.
199 Sasahara, I. et al. (2020) l-Serine and EPA relieve chronic low-back and knee pain in adults: a randomized, double-blind, placebo-controlled trial. J. Nutr., 150, 2278–2286.
200 Lustberg, M. B. et al. (2018) Randomized placebo-controlled pilot trial of omega 3 fatty acids for prevention of aromatase inhibitor-induced musculoskeletal pain. Breast Cancer Res. Treat., 167, 709–718.
201 Hershman, D. L. et al. (2015) Randomized multicenter placebo-controlled trial of omega-3 fatty acids for the control of aromatase inhibitor-induced musculoskeletal pain: SWOG S0927. J. Clin. Oncol., 33, 1910–1917.
202 Ruiz-Tovar, J. et al. (2019) Preoperative administration of Omega-3 fatty acids on postoperative pain and acute-phase reactants in patients undergoing Roux-en-Y gastric bypass: A randomized clinical trial. Clin. Nutr., 38, 1588–1593.
203 Bernabe-Garcia, M. et al. (2016) Enteral docosahexaenoic acid reduces analgesic administration in neonates undergoing cardiovascular surgery. Ann. Nutr. Metab., 69, 150–160.
204 Zafari, M., Behmanesh, F. & Agha Mohammadi, A. (2011) Comparison of the effect of fish oil and ibuprofen on treatment of severe pain in primary dysmenorrhea. Casp. J. Intern. Med., 2, 279–282.
205 Murina, F., Graziottin, A., Felice, R. & Gambini, D. (2017) Alpha lipoic acid plus omega-3 fatty acids for vestibulodynia associated with painful bladder syndrome. J. Obstet. Gynaecol. Can., 39, 131–137.
206 Polycystic ovary syndrome (2019) www.nhs.uk/conditions/ https://www.nhs.uk/conditions/polycystic-ovary-syndrome-pcos/.
207 Sadeghi, A., Djafarian, K., Mohammadi, H. & Shab-Bidar, S. (2017) Effect of omega-3 fatty acids supplementation on insulin resistance in women with polycystic ovary syndrome: Meta-analysis of randomized controlled trials. Diabetes Metab. Syndr., 11, 157–162.
208 Yang, K., Zeng, L., Bao, T. & Ge, J. (2018) Effectiveness of Omega-3 fatty acid for polycystic ovary syndrome: a systematic review and meta-analysis. Reprod. Biol. Endocrinol., 16, 27.
209 von Schacky, C. (2020) Omega-3 fatty acids in pregnancy—the case for a target Omega-3 Index. Nutrients, 12, 898.
210 Hoge, A. et al. (2020) Impact of erythrocyte long-chain omega-3 polyunsaturated fatty acid levels in early pregnancy on birth outcomes: findings from a Belgian cohort study. J. Perinatol., 40, 488–496.
211 Kitamura, Y. et al. (2018) Fatty acid composition of the erythrocyte membranes varies between early-term, full-term, and late-term infants in Japan. Ann. Nutr. Metab., 73, 335–343.
212 Markhus, M. W. et al. (2013) Low omega-3 index in pregnancy is a possible biological risk factor for postpartum depression. PLoS One, 8, e67617.
213 Jiang, L. et al. (2020) Omega-3 fatty acids plus vitamin for women with gestational diabetes or prediabetes: a meta-analysis of randomized controlled studies. J. Matern. Fetal. Neonatal Med., 1–8. doi:10.1080/14767058.2020.1814239.
214 Zhong, N. & Wang, J. (2019) The efficacy of omega-3 fatty acid for gestational diabetes: a meta-analysis of randomized controlled trials. Gynecol. Endocrinol., 35, 4–9.
215 Gao, L. et al. (2020) The impact of omega-3 fatty acid supplementation on glycemic control in patients with gestational diabetes: a systematic review and meta-analysis of randomized controlled studies. J. Matern. Fetal Neonatal Med., 33, 1767–1773.
216 Wei-Hong, L., Cheng-Gui, Z., Peng-Fei, G., Heng, L. & Jian-Fang, Y. (2017) Omega-3 fatty acids as monotherapy in treating depression in pregnant women: a meta-analysis of randomized controlled trials. Iran. J. Pharm. Res. IJPR, 16, 1593–1599.
217 Lin, P.-Y., Chang, C.-H., Chong, M. F.-F., Chen, H. & Su, K.-P. (2017) Polyunsaturated fatty acids in perinatal depression: a systematic review and meta-analysis. Biol. Psychiatry, 82, 560–569.
218 Suradom, C., Suttajit, S., Oon-Arom, A., Maneeton, B. & Srisurapanont, M. (2021) Omega-3 polyunsaturated fatty acid (n-3 PUFA) supplementation for prevention and treatment of perinatal depression: a systematic review and meta-analysis of randomized-controlled trials. Nord. J. Psychiatry, 75, 239–246.
219 Mocking, R. J. T. et al. (2020) Omega-3 fatty acid supplementation for perinatal depression: a meta-analysis. J. Clin. Psychiatry, 81, 19r13106.
220 Zhang, M.-M. et al. (2020) The efficacy and safety of omega-3 fatty acids on depressive symptoms in perinatal women: a meta-analysis of randomized placebo-controlled trials. Transl. Psychiatry, 10, 193.
221 Middleton, P. et al. (2018) Omega-3 fatty acid addition during pregnancy. Cochrane Database Syst. Rev., 11, CD003402.
222 Serra, R. et al. (2021) Supplementation of omega 3 during pregnancy and the risk of preterm birth: a systematic review and meta-analysis. Nutrients, 13, 1704.
223 Matsumura, K. et al. (2017) Effects of omega-3 polyunsaturated fatty acids on psychophysiological symptoms of post-traumatic stress disorder in accident survivors: A randomized, double-blind, placebo-controlled trial. J. Affect. Disord., 224, 27–31.
224 Zeev, K., Michael, M., Ram, K. & Hagit, C. (2005) Possible deleterious effects of adjunctive omega-3 fatty acids in post-traumatic stress disorder patients. Neuropsychiatr. Dis. Treat., 1, 187–190.
225 Brasky, T. M. et al. (2013) Plasma phospholipid fatty acids and prostate cancer risk in the SELECT Trial. JNCI J. Natl. Cancer Inst., 105, 1132–1141.
226 Haas-Haseman, M. (2015) Weighing the benefits of fish oil for patients with prostate cancer: a subcohort review from the SELECT Trial. J. Adv. Pract. Oncol., 6, 376–378.
227 Aucoin, M. et al. (2017) Fish-derived omega-3 fatty acids and prostate cancer: a systematic review. Integr. Cancer Ther., 16, 32–62.
228 Dinwiddie, M. T., Terry, P. D., Whelan, J. & Patzer, R. E. (2016) Omega-3 fatty acid consumption and prostate cancer: a review of exposure measures and results of epidemiological studies. J. Am. Coll. Nutr., 35, 452–468.
229 Alexander, D. D. et al. (2015) Meta-analysis of long-chain omega-3 polyunsaturated fatty acids (LCω-3PUFA) and prostate cancer. Nutr. Cancer, 67, 543–554.
230 Fu, Y.-Q., Zheng, J.-S., Yang, B. & Li, D. (2015) Effect of individual omega-3 fatty acids on the risk of prostate cancer: a systematic review and dose-response meta-analysis of prospective cohort studies. J. Epidemiol., 25, 261–274.
231 Dai, Y. & Liu, J. (2021) Omega-3 long-chain polyunsaturated fatty acid and sleep: a systematic review and meta-analysis of randomized controlled trials and longitudinal studies. Nutr. Rev., 79, 847−868. doi:10.1093/nutrit/nuaa103.
232 Patan, M. J. et al. (2021) Differential effects of DHA- and EPA-rich oils on sleep in healthy young adults: a randomized controlled trial. Nutrients, 13, 248.
233 Jahangard, L. et al. (2018) Influence of adjuvant omega-3-polyunsaturated fatty acids on depression, sleep, and emotion regulation among outpatients with major depressive disorders – Results from a double-blind, randomized and placebo-controlled clinical trial. J. Psychiatr. Res., 107, 48–56.
234 Chang, C.-H. et al. (2018) Safety and tolerability of prescription omega-3 fatty acids: A systematic review and meta-analysis of randomized controlled trials. Prostaglandins Leukot. Essent. Fatty Acids, 129, 1–12.
235 Begtrup, K. M., Krag, A. E. & Hvas, A.-M. (2017) No impact of fish oil supplements on bleeding risk: a systematic review. Dan. Med. J., 64, A5366.
236 Kelley, D. S. & Rudolph, I. L. (2000) Effect of individual fatty acids of ω-6 and ω-3 type on human immune status and role of eicosanoids. Nutrition, 16, 143–145.
237 European Food Safety Authority (EFSA) (2012) Scientific opinion on the tolerable upper intake level of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA). EFSA J., 10, 2815.
238 Fernandes, A. R., Rose, M., White, S., Mortimer, D. N. & Gem, M. (2006) Dioxins and polychlorinated biphenyls (PCBs) in fish oil dietary supplements: occurrence and human exposure in the UK. Food Addit. Contam., 23, 939–947.
239 Blanco, L., Martínez, A., Ferreira, M., Vieites, J. & Cabado, A. (2013) Polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) and dioxin-like polychlorinated biphenyls (dl-PCBs) in fish, seafood products and fish oil in Spain. Food Addit. Contam. Part B, Surveill., 6, 218–230.
240 Gouvêa, H., Paula, D., Silva, T., Campos, A. & Ito, M. (2019) Fatty acid content, oxidation markers and mercury in fish oil supplements commercialized in Brasília, Brazil. Orbital Electron. J. Chem., 11.