By Beverly Gibbs and Benjamin I. Brown
Abstract
A number of patients with ulcerative colitis (UC) fail to achieve clinical remission with standard treatments, and may become less responsive to these treatments over time. Butyrate, a short-chain fatty acid, plays a major role in the immune homeostasis of the colonic mucosa, and oral butyrate has shown some promise as an adjuvant therapy in a small number of clinical studies, including for treatment-resistant patients. This case report describes an individual with a diagnosis of UC resistant to pharmacological and nutritional interventions who responded well to a trial of oral butyrate. Butyrate appears to be a promising therapy for UC, but questions around its efficacy, personalisation and safety require further investigation.
Cite as: Gibbs, B. & Brown, B. (2021) Butyrate therapy for treatment-resistant ulcerative colitis: a case study. Nutr. Med. J., 1 (1), 60-67.
Affiliation: B. Gibbs was in private practice and is now retired. B. Brown is with the Nutritional Medicine Institute, London, UK.
Corresponding author: Benjamin I. Brown (email: ben@nmi.health).
Article history: Received 21 April 2021; Peer-reviewed and received in revised form 11 March 2022; Accepted 12 March 2022. Available online 31 March 2022.
Published by: The Nutritional Medicine Institute
Open Access: This is an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs licence (http:// creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reproduction and distribution of the work, in any medium, provided the original work is not altered or transformed in any way, and that the work is properly cited. For commercial use please contact support@nmi.health
Introduction
Inflammatory bowel diseases (IBDs), including ulcerative colitis (UC), have a high prevalence in developed countries.1 In the UK, the prevalence of UC is approximately 243 per 100 000, equivalent to 146 000 people living with UC.2 The treatment of IBDs is primarily based on pharmacological interventions, in particular aminosalicylates, corticosteroids, immunomodulators, immunosuppressants and biologics; however, a significant number of patients fail to achieve clinical remission and may become less responsive to these treatments over time.3 For example, sulfasalazine and 5-aminosalicylates have an expected remission rate of about 50%.4 And, independent of treatment response, 90% of people with UC will experience a relapsing course of their illness.5 More effective and safer management approaches are needed and may be aided by improved understanding of the disease pathophysiology.6 One possible approach is the personalisation of a wider range of candidate therapies, not only pharmacological interventions, based on an individual’s unique environmental exposures and phenotypic expression.7
Ulcerative colitis is a chronic inflammatory disease characterised by mucosal inflammation that is thought to be the result of several contributing factors, including genetic susceptibility, a dysregulated immune response, altered gut microbiota and various environmental factors.8 Although UC is a heterogenous disease, one possible disease pathway that transverses environmental, genetic, microbial and immunological influences is altered butyrate utilisation.9 Butyrate is a short-chain fatty acid (SCFA), mainly produced by the fermentation of dietary fibres by butyrate-producing bacteria, and plays a major role in the physiology and immune homeostasis of the colonic mucosa.10
Butyrate production and metabolism may be impaired in patients with UC. Production of butyrate may be reduced due to a low-fibre diet,11 although it has been observed that there are large individual differences in butyrate synthesis in response to fibre, likely due to the intrinsic butyrate-producing capacity of a person’s resident bacteria.12 Indeed, patients with UC have generally been found to have low levels of butyrate-synthesising bacterial species such as Roseburia and Faecalibacterium prausnitzii.13 Also, stool samples of patients with IBDs have generally found low levels of butyrate when compared with healthy controls.14,15,16,17
Metabolism of butyrate may be impaired in the colonocytes of patients with IBD, with reduced butyrate transport limiting uptake and resulting in cellular butyrate deficiency.18 This metabolic issue suggests that dietary fibre and butyrate-synthesising bacteria may not always be related to low butyrate levels. In support of this, a study in patients with active IBD found that decreased butyrate concentrations were not related to lower stool content of butyrate-producing bacteria.19
Butyrate supplementation has been explored as a management approach in IBDs, and would be of particular interest if it can overcome poor response to increased dietary fibre intake, low stool content of butyrate-producing bacteria, and/or impaired butyrate transport and metabolism, discussed above. Treatment with oral butyrate salts in combination with mesalazine has yielded promising results. Patients (n = 12) with UC who received butyrate (4 g/day) plus mesalazine for 6 weeks reduced disease histology and symptoms scores better than mesalazine alone.20 In another report, patients with UC (n = 216) with a poor response to mesalazine had a marked improvement of symptoms and in the endoscopic appearance of mucosa with the addition of 921 mg butyrate and 750 mg inulin.21 Also, butyrate with inulin plus mesalazine in active UC (n = 37) resulted in an 85% response rate compared with 55% with mesalazine alone.22 The butyrate and inulin group also had improved capacity for butyrate synthesis by the gut microbiota, and lowered serum inflammatory biomarkers.20
A potential mechanism of action of butyrate was explored in a clinical trial of patients with IBD. In this study, oral butyrate (1800 mg/day) or placebo was administered with conventional therapy for 2 months, and clinical disease activity, quality of life and faecal microbiota from stool samples were assessed by 16S sequencing. Oral butyrate increased SCFA-producing bacteria in patients with UC (Lachnospiraceae spp.) and the butyrate-producing bacteria in patients with Crohn’s disease (Butyricicoccus).23 However, no change in disease activity was observed in this study.
The evidence suggests that some patients with IBD could have a significant clinical response to oral butyrate therapy. The following case report describes an individual with a diagnosis of UC resistant to pharmacological and nutritional interventions who responded well to a trial of oral butyrate under the care of a Nutritional Therapist. In the UK, Nutritional Therapy is an evidence-based practice that utilises dietary therapies, nutrient-based supplements and lifestyle medicine to improve underlying pathophysiology in an integrative, personalised and patient-centred model of care.24
Case presentation
Presenting concerns
The case was a 62-year-old male, non-smoker, with a 25-year history of UC. His primary complaint was frequent, loose bowel movements, with urgency and incomplete evacuation.
Case history
For a number of years following diagnosis, remission had been obtained through aminosalicylate treatment (Mezavant) with flare-ups occurring approximately annually, treated with corticosteroids at doses of up to 50 mg/day. The case was seen regularly at an IBD clinic, under the care of a consultant gastroenterologist.
In recent years, flare-ups had increased in frequency to approximately every 6 months, and then more recently (since 2019) to every 3 months. Each time, oral steroids were prescribed to reduce symptoms, with aminosalicylate treatment for maintenance and prednisolone steroid enemas at the first sign of a recurrence (with the aim of minimising systemic steroids).
In 2012, immunomodulator therapy was trialled (azathioprine and mercaptopurine), but this was poorly tolerated, with the case reporting severe nausea, lethargy and low white cell count. In the same year, the case suffered a herpes zoster (shingles) infection, resulting in 6 weeks absence from work. UC treatment then reverted to maximum doses of Mezavant and oral steroids as required.
Various dietary interventions were tried in this period, including gluten-free (dietary gluten avoidance), dairy-free (dietary dairy avoidance), specific carbohydrate diet (a restrictive, grain-free diet plan developed for the management of IBD) and low-FODMAP diet (an elimination diet used to help manage the symptoms of irritable bowel syndrome). Small amounts of benefit were obtained with each (e.g. more energy, slightly reduced frequency and urgency), but none gave sustained relief of symptoms. Supplementation with various probiotics over this period (e.g. VSL#3) and prebiotics also appeared to give no relief.
The case undertook regular monitoring by an NHS IBD clinic, and was assessed by endoscopy and biopsy at intervals.
Side-effects suffered from corticosteroids
In the meantime, the case was diagnosed with corticosteroid-induced osteoporosis in the spine and was prescribed AdCal (combination calcium and vitamin D). Weekly bisphosphonates (alendronic acid) were also commenced at 70 mg.
In July 2015 the case was hospitalised for an unresponsive disease flare-up that had resulted in a 10 kg weight loss. The case was treated with very-high-dose steroids intravenously for 24 hours, being discharged to continue oral treatment the next day. Following this experience, the case decided to limit working hours to part-time because of reduced quality of life.
The option of surgery was discussed in 2018, but the decision was made to defer this as the last possible resort.
In March 2019, the case suffered a 5-mm calcium oxalate kidney stone, which was passed naturally (but with severe pain) in July 2019 without further treatment. He was then advised to stop taking AdCal by his physician as this could have been the cause.
In May 2020, the case experienced the start of the latest flare-up, following a short period of remission of 3 months. Sleep was disturbed in this period also. His physician decided to manage this conservatively with additional steroid enemas daily due to the osteoporotic side-effects of oral treatment, but only partial relief was obtained.
Unresponsive to treatment
In June 2020, the case, still on Mezavant, was prescribed oral corticosteroids once more, but this did not resolve symptoms.
In August 2020, funding was obtained by the IBD clinic for treatment with the biologic tumour necrosis factor (TNF)-alpha inhibitor Imraldi (40 mg once every 2 weeks), administered by self-injection. After some promising indications of possible relief, the case became unresponsive within weeks, and a sigmoidoscopy in November 2020 confirmed the following symptoms:
• redness in the distal colon;
• loss of vascular pattern;
• patchy erosions.
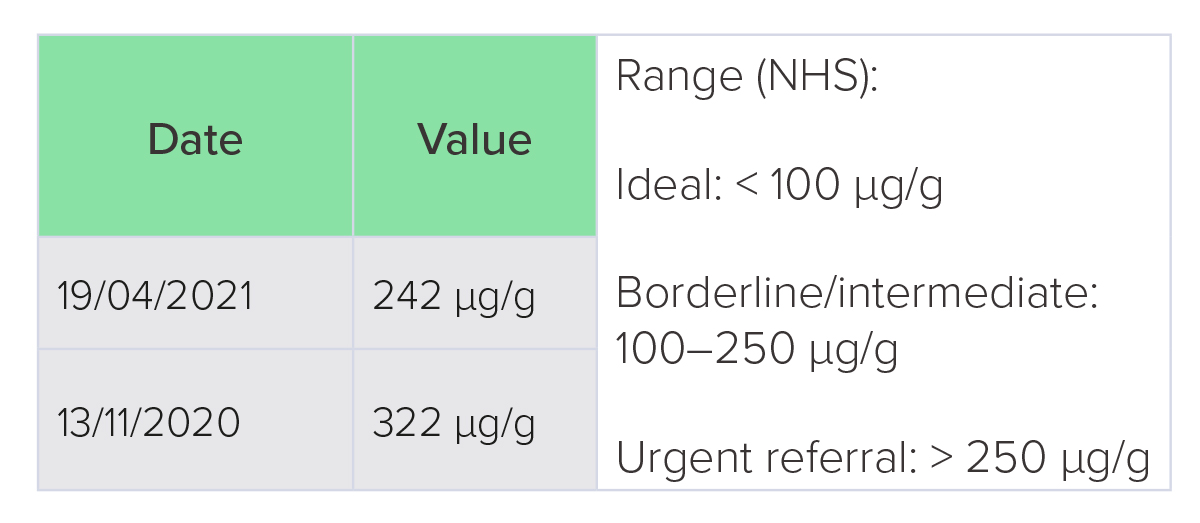
Faecal calprotectin at this time was 322 µg/g (ideal level < 100 µg/g) despite steroid treatment (Table 1). A more frequent dose of Imraldi (40 mg once a week) was tried at this point, but without success.
In December 2020, the IBD clinic advised the next stage of treatment would be vedolizumab, a ‘gut-selective integrin blocker’ that targets white blood cells, delivered by intravenous infusion, for which funding approval would be sought. The suggested response rate of this medication was 40−45%.
Intervention
Before this latest treatment commenced, the case’s Nutritional Therapist became aware of the potential use of butyrate supplementation for symptomatic relief of UC.
The case commenced oral calcium magnesium butyrate at a dose of 1.2 g per day, whilst continuing Mezavant treatment at 4.8 g/day.
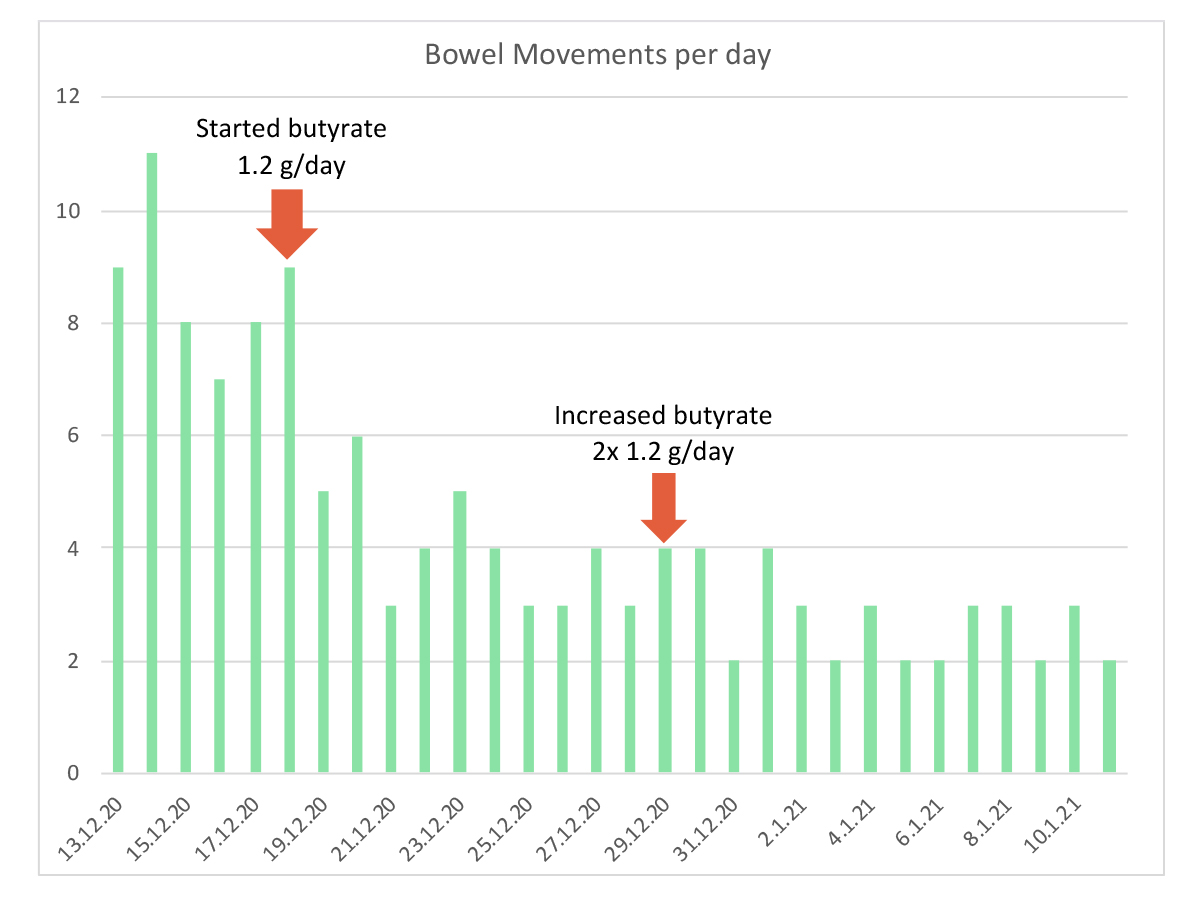
Within 2 days, the case felt a positive response to butyrate, and within 4 days the frequency of bowel movements had reduced from eight−11 per day to four−six per day, with less urgency.
Eleven days later, the butyrate dose was increased to 2.4 g per day (in two divided doses) and there was further improvement to two−four bowel movements per day, with normal stool and feeling of complete evacuation.
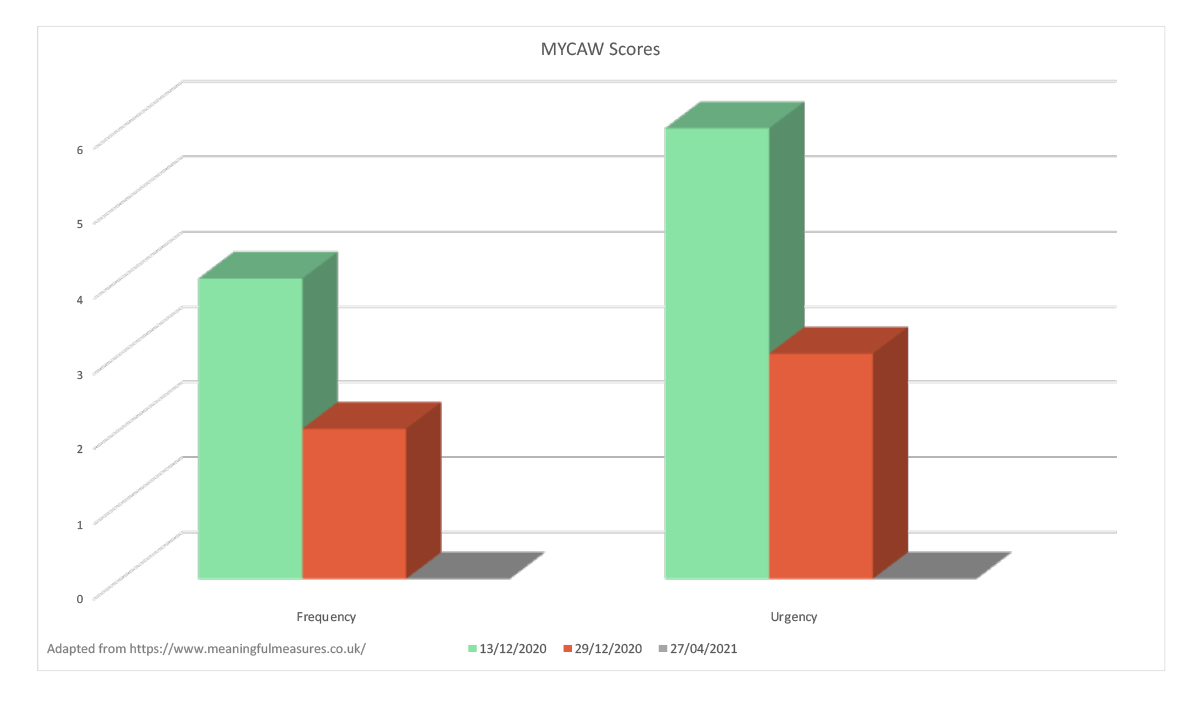
From January 2021 to the present day, stool frequency has stabilised at two−three per day (Figure 1) with no feelings of urgency (Figure 2). The case reports that bowel function feels much better, even compared with the effects of previous symptom alleviation from corticosteroids. Faecal calprotectin
is reported to have reduced to 242 µg/g.
Figure 1: Number of bowel movements per day (1−12).
Figure 2: Improvement in symptom scores.
Self-reported MYCAW scores prior to treatment (13/12/3020) to post-treatment (29/12/20) and present day (27/04/21). MYCAW is an individualised questionnaire designed for evaluating holistic and personalised approaches to supporting people.25
Progress to date
The case reports continued relief of symptoms from combined Mezavant and butyrate. The butyrate supplement is well tolerated and no side-effects have been reported; the pungent smell from butyric acid is not a deterrent to this case and does not linger on the breath.
The case has been able to exercise at moderate−high intensity for 1−2 hours at a time on a regular basis, whereas previously the urgency for a bowel movement would have hampered this. Quality of life has returned, and the case is able to tolerate a wider variety of foods, maintaining a largely pescatarian, wholefoods diet.
An appointment with the consultant gastroenterologist in July 2021 to discuss the case’s condition resulted in an agreement to continue oral butyrate as maintenance therapy. Body weight is stable at 73 kg (body mass index 23 kg/m2).
Table 1: Faecal calprotectin test results
Discussion
Butyrate represents an emerging novel therapy for UC that could be used in a personalised way for cases who have a poor response to standard management approaches, including pharmacotherapy, probiotics, prebiotics and dietary therapy, as supported by a small number of exploratory studies,18,19,20 and the case described here. Important but unanswered questions remain that could help clarify the role and use of butyrate. Firstly, could biomarkers be used to predict candidates for therapy? Butyrate-synthesising bacterial species and butyrate can be assessed clinically with stool analysis, but it is currently unknown if they can be used to predict treatment response. The high level of individual variability in microbial markers and metabolism could mean that such biomarkers may not be reliable. Microbiome testing is fraught with important limitations that impair translation to clinical practice, including the high variability and instability of an individual’s microbiome, unpredictable response to nutritional interventions, and important variations in laboratory methods and results, all of which impact reliability and usefulness of test data.26 Similarly, assessment of butyrate in stool samples is limited by significant individual variability, variation from day to day, and the time of day of the sample.27
Secondly, what is the optimal dose of butyrate? Currently there are few clinical studies and no dose−response studies to inform dose regimes. It is plausible that doses higher than those used to date (up to 4 g/day in UC) would be more effective and have relevance for active UC. One analysis suggests that, based on daily butyrate production and requirements, higher doses (up to 10 g/day) may be optimal.28 Dosage regimes need further exploration, including the assessment of higher doses for active disease, and the optimal dose regimes for supporting maintenance of remission.
Thirdly, it would be interesting to understand the potential for butyrate as a monotherapy. Clinical research to date suggests it may be better suited to use as an adjuvant to pharmacotherapy, and possibly prebiotics, probiotics and nutritional interventions, but its use as a monotherapy has not yet been explored in UC, and given butyrate’s potential safety it may be an interesting candidate for maintaining IBD remission.29 This case report suggests it may be useful for maintaining UC in remission, but this requires validation with further study.
Finally, although butyrate is generally considered very safe and well tolerated, rigorous safety assessment is warranted if butyrate is to have more widespread use.30 Evidence to date does suggest a high degree of safety with no toxicity, side-effects, nutrient−drug interactions or safety concerns from human clinical trials in patients with IBD and other gastrointestinal disorders, but this requires more rigorous assessment.28
Conclusion
This case report suggests a novel role for oral butyrate therapy. In a case of UC that was resistant to conventional therapy and nutritional interventions, butyrate administration appeared to result in significant control of symptoms and maintenance of remission. It is hypothesised that this may be due to exogenous butyrate supporting an individual’s higher metabolic demand for endogenous butyrate that could not be met due to impaired butyrate synthesis and/or increased metabolic requirement, although this theory requires validation with more research. Evidence to date suggests that butyrate may be a useful, safe therapy that could complement the usual care of IBD.
Acknowledgements
Author contributions: B. Gibbs compiled the case report. B. Brown drafted the manuscript Introduction, Discussion and Conclusions on consultation with B. Gibbs. B. Gibbs and B. Brown reviewed and agreed on the final manuscript.
Peer-reviewers and editors: the Nutritional Medicine Institute thanks the peer-reviewers and editors for their important contributions.
Funding: No funding was received for this work.
Declaration of interest: B. Brown has received consultancy fees from Pure Encapsulations, Sudbury, MA, USA. B. Gibbs has no interests to declare.
.
References
1 Ng, S. C. et al. (2017) Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet, 390 (10 114), 2769−2778.
2 Tun, G. S., Harris, A. & Lobo, A. J. (2017) Ulcerative colitis: management in adults, children and young people − concise guidance. Clin. Med. (Lond)., 17 (5), 429−433.
3 Cai, Z., Wang, S. & Li, J. (2021) Treatment of inflammatory bowel disease: a comprehensive review. Front. Med. (Lausanne), 8, 765 474.
4 Danese, S. & Fiocchi, C. (2011) Ulcerative colitis. N. Engl. J. Med., 365 (18), 1713−1725.
5 Langholz. E. et al. (1994) Course of ulcerative colitis: analysis of changes in disease activity over years. Gastroenterology, 107, 3−11.
6 Yadav, V., Varum, F., Bravo, R., Furrer, E., Bojic, D. & Basit, A. W. (2016) Inflammatory bowel disease: exploring gut pathophysiology for novel therapeutic targets. Transl. Res., 176, 38−68.
7 de Souza, H. S. P. & Fiocchi, C. (2018) Network medicine: a mandatory next step for inflammatory bowel disease. Inflamm. Bowel Dis., 24 (4), 671−679.
8 Kobayashi, T. et al. (2020) Ulcerative colitis. Nat. Rev. Dis. Primers, 6 (1), 74.
9 Parada Venegas, D. et al. (2019) Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol., 10, 277. Erratum in: (2019) Front. Immunol., 10, 1486.
10 Silva, J. P. B. et al. (2018) Protective mechanisms of butyrate on inflammatory bowel disease. Curr. Pharm. Des., 24 (35), 4154−4166.
11 Hallert, C., Björck, I., Nyman, M., Pousette, A., Grännö, C. & Svensson, H. (2003) Increasing fecal butyrate in ulcerative colitis patients by diet: controlled pilot study. Inflamm. Bowel Dis., 9 (2), 116−121.
12 Baxter, N. T., Schmidt, A. W., Venkataraman, A., Kim, K. S., Waldron, C. & Schmidt, T. M. (2019) Dynamics of human gut microbiota and short-chain fatty acids in response to dietary interventions with three fermentable fibers. mBio, 10 (1), e02566-18.
13 Laserna-Mendieta, E. J. et al. (2018) Determinants of reduced genetic capacity for butyrate synthesis by the gut microbiome in Crohn’s disease and ulcerative colitis. J. Crohns Colitis, 12 (2), 204−216.
14 Huda-Faujan, N. et al. (2010) The impact of the level of the intestinal short chain fatty acids in inflammatory bowel disease patients versus healthy subjects. Open Biochem. J., 4, 53−58.
15 Marchesi, J. R. et al. (2007) Rapid and noninvasive metabonomic characterization of inflammatory bowel disease. J. Proteome Res., 6 (2), 546−551.
16 Bjerrum, J. T. et al. (2015) Metabonomics of human fecal extracts characterize ulcerative colitis, Crohn’s disease and healthy individuals. Metabolomics, 11, 122−133.
17 Machiels, K. et al. (2014) A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut, 63 (8), 1275−1283.
18 Thibault, R., Blachier, F., Darcy-Vrillon, B., de Coppet, P., Bourreille, A. & Segain, J. P. (2010) Butyrate utilization by the colonic mucosa in inflammatory bowel diseases: a transport deficiency. Inflamm. Bowel Dis., 16 (4), 684−695.
19 Ferrer-Picón, E. et al. (2020) Intestinal inflammation modulates the epithelial response to butyrate in patients with inflammatory bowel disease. Inflamm. Bowel Dis., 26 (1), 43−55.
20 Vernia, P. et al. (2000) Combined oral sodium butyrate and mesalazine treatment compared to oral mesalazine alone in ulcerative colitis: randomized, double-blind, placebo-controlled pilot study. Dig. Dis. Sci., 45 (5), 976−981.
21 Assisi, R. F. and GISDI Study Group (2008) Combined butyric acid/mesalazine treatment in ulcerative colitis with mild-moderate activity. Results of a multicentre pilot study. Minerva Gastroenterol. Dietol., 54 (3), 231−238.
22 Sitkin, S., Vakhitov, T. & Pokrotnieks, J. (2018) How to increase the butyrate-producing capacity of the gut microbiome: do IBD patients really need butyrate replacement and butyrogenic therapy? J. Crohns Colitis, 12 (7), 881−882.
23 Facchin, S. et al. (2020) Microbiota changes induced by microencapsulated sodium butyrate in patients with inflammatory bowel disease. Neurogastroenterol. Motil., 32 (10), e13914.
24 Harris, M. D. & Benbow, A. (2021) Evaluating the effectiveness of nutritional therapy in the McClelland Teaching Clinic at the University of Worcester. Online J. Complement. Alt. Med., 6 (3), 2021.
25 Paterson, C., Thomas, K., Manasse, A., Cooke, H. & Peace, G. (2007) Measure Yourself Concerns and Wellbeing (MYCaW): an individualised questionnaire for evaluating outcome in cancer support care that includes complementary therapies. Complement. Ther. Med., 15 (1), 38−45.
26 Allaband, C, et al. (2019) Microbiome 101: studying, analyzing, and interpreting gut microbiome data for clinicians. Clin. Gastroenterol. Hepatol.,. 17 (2), 218−230.
27 Jones, J., Reinke, S. N., Ali, A., Palmer, D. J. & Christophersen, C. T. (2021) Fecal sample collection methods and time of day impact microbiome composition and short chain fatty acid concentrations. Sci. Rep., 11 (1), 13 964.
28 Banasiewicz, T., Domagalska, D., Borycka-Kiciak, K. & Rydzewska, G. (2020) Determination of butyric acid dosage based on clinical and experimental studies − a literature review. Prz. Gastroenterol., 15 (2), 119−125.
29 van der Beek, C. M., Dejong, C. H. C., Troost, F. J. , Masclee, A. A. M. & Lenaerts, K. (2017) Role of short-chain fatty acids in colonic inflammation, carcinogenesis, and mucosal protection and healing. Nutr. Rev., 75 (4), 286−305.
30 Deleu, S., Machiels, K., Raes, J., Verbeke, K. & Vermeire, S. (2021) Short chain fatty acids and its producing organisms: an overlooked therapy for IBD? EBioMedicine, 66, 103 293.